Abstract
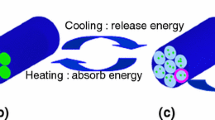
In order to adjust buildings temperature, a dodecanol (DD)-palmitic acid (PA)/hydroxylpropyl methyl cellulose (HPMC) composite phase change material was prepared by vacuum impregnation. DD-PA was absorbed into the HPMC, which was verified by specific surface area, pore size (BET) and scanning electron microscopy (SEM) analysis. The results of Fourier transform infrared spectroscopy (FTIR) and X-ray diffractometer (XRD) indicated that the HPMC and DD-PA were only physical combination. The differential scanning calorimetry (DSC) analysis revealed that the phase change temperature and latent heat were 19.34 ºC and 113.12 J g−1, which means good energy storage capacity. The 5% mass loss temperatures (T-5%) of thermo-gravimetric analysis (TG) was higher than 50 ºC, showing the good thermal stability of composite phase change materials. About 60% DD-PA was absorbed in HPMC, which detected by DSC and TG. After 100 thermal cycling, the latent heat, onset temperature (T0), peak temperature (Tpeak) and end temperature (Tend) had changed −1.02%, −8.20%, −4.29% and −5.60%. The result showed that the composite phase change materials own good thermal reliability. In addition, the 2% multi-walled carbon nanotubes (MW CNTs) were added to improve the thermal conductivity. And the thermal conductivity was increased from 0.130 to 0.172 W (mK)−1, the total thermal storage-release time was decreased from 3740 to 2310 s.












Similar content being viewed by others
References
Xu Y, Li MJ, Zheng ZJ, Xue XD. Melting performance enhancement of phase change material by a limited amount of metal foam: Configurational optimization and economic assessment. Appl Energ. 2018;212:868–80.
Yuan P, Zhang P, Liang T, Zhai SP, Yang DG. Effects of functionalization on energy storage properties and thermal conductivity of graphene/n-octadecane composite phase change materials. J Mater Sci. 2019;54(2):1488–501.
Masoumi H, Khoshkhoo RH, Mirfendereski SM. Modification of physical and thermal characteristics of stearic acid as a phase change materials using TiO2-nanoparticles. Thermochim Acta. 2019;675:9–17.
Sheng N, Dong KX, Zhu CY, Akiyama T, Nomura T. Thermal conductivity enhancement of erythritol phase change material with percolated aluminum filler. Mater Chem Phys. 2019;229:87–91.
Tang YJ, Jia YT, Alva G, Huang X, Fang GY. Synthesis, characterization and properties of palmitic acid/high density polyethylene/graphene nanoplatelets composites as form-stable phase change materials. Sol Energ Mat Sol C. 2016;155:421–9.
Tang BT, Wu C, Qiu MG, Zhang XW, Zhang SF. PEG/SiO2-Al2O3 hybrid form-stable phase change materials with enhanced thermal conductivity. Mater Chem Phys. 2014;144(1–2):162–7.
Peng SQ, Huang J, Wang TY, Zhu PP. Effect of fumed silica additive on supercooling thermal reliability and thermal stability of Na2HPO4 center dot 12H2O as inorganic PCM. Thermochim Acta. 2019;675:1–8.
Temel UN, Kurtulus S, Parlak M, Yapici K. Size-dependent thermal properties of multi-walled carbon nanotubes embedded in phase change materials. J Therm Anal Calorim. 2018;132:631–41.
Wang W, Wang CY, Wang T, Li W, Chen LJ, Zou RG, Zheng J, Li XG. Enhancing the thermal conductivity of n-eicosane/silica phase change materials by reduced graphene oxide. Mater Chem Phys. 2014;147(3):701–6.
Qian YC, Zhang Y, Sun JH, Song C, Jing Y, Shao F, Jia YZ, Tao ZY, Wang XQ, Liu H. The efect of hydrophilic modifcation of expanded graphite on the thermophysical properties of magnesium chloride hexahydrate. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08942-x.
Hohlein S, Konig-Haagen A, Bruggemann D. Thermophysical Characterization of MgCl2 center dot 6H(2)O, Xylitol and Erythritol as Phase Change Materials (PCM) for Latent Heat Thermal Energy Storage (LHTES). Materials. 2017;10(4):444. https://doi.org/10.3390/ma10040444.
Wu B, Zhao Y, Liu Q, Zhou CL, Zhang X, Lei JX. Form-stable phase change materials based on castor oil and palmitic acid for renewable thermal energy storage. J Therm Anal Calorim. 2019;137:1225–32.
Han LP, Ma GX, Xie SL, Sun JH, Jia YZ, Jing Y. Preparation and characterization of the shape-stabilized phase change material based on sebacic acid and mesoporous MCM-41. J Therm Anal Calorim. 2017;130:935–41.
Ramakrishnan S, Wang XM, Sanjayan J. Thermal enhancement of paraffin/hydrophobic expanded perlite granular phase change composite using graphene nanoplatelets. Energ Buildings. 2018;169:206–15.
Cheng XM, Li G, Yu GM, Li YY, Han JQ. Effect of expanded graphite and carbon nanotubes on the thermal performance of stearic acid phase change materials. J Mater Sci. 2017;52(20):12370–9.
Ziapour BM, Hashtroudi A. Performance study of an enhanced solar greenhouse combined with the phase change material using genetic algorithm optimization method. Appl Therm Eng. 2017;110:253–64.
Lee KO, Medina MA, Sun XQ, Jin X. Thermal performance of phase change materials (PCM)-enhanced cellulose insulation in passive solar residential building walls. Sol Energ. 2018;163:113–21.
Chalco-Sandoval W, Fabra MJ, Lopez-Rubio A, Lagaron JM. Use of phase change materials to develop electrospun coatings of interest in food packaging applications. J Food Eng. 2017;192:122–8.
Cheng WL, Mei BJ, Liu YN, Huang YH, Yuan XD. A novel household refrigerator with shape-stabilized PCM (Phase Change Material) heat storage condensers: An experimental investigation. Energy. 2011;36(10):5797–804.
Wu B, Fu WX, Kong BW, Hu K, Zhou CL, Lei JX. Preparation and characterization of stearic acid/polyurethane composites as dual phase change material for thermal energy storage. J Therm Anal Calorim. 2018;132:907–17.
Sari A, Karaipekli A, Alkan C. Preparation, characterization and thermal properties of lauric acid/expanded perlite as novel form-stable composite phase change material. Chem Eng J. 2009;155(3):899–904.
Zhang D, Zhou HM, Wu K, Li ZJ. Granular phase changing composites for thermal energy storage. Sol Energy. 2005;78(3):471–80.
Karaipekli A, Sari A. Capric-myristic acid/expanded perlite composite as form-stable phase change material for latent heat thermal energy storage. Renew Energ. 2008;33(12):2599–605.
Zhu N, Liu PP, Liu FL, Hu PF, Wu MD. Energy performance of double shape-stabilized phase change materials wallboards in office building. Appl Therm Eng. 2016;105:180–8.
Delcroix B, Kummert M, Daoud A. Development and numerical validation of a new model for walls with phase change materials implemented in TRNSYS. J Build Perform Simu. 2017;10(4):422–37.
Xia Y, Zhang XS. Experimental research on a double-layer radiant floor system with phase change material under heating mode. Appl Therm Eng. 2016;96:600–6.
Xia YP, Cui WW, Zhang HZ, Zou YJ, Xiang CL, Chu HL, Qiu SJ, Xu F, Sun LX. Preparation and thermal performance of n-octadecane/expanded graphite composite phase-change materials for thermal management. J Therm Anal Calorim. 2018;131:81–8.
Kumar R, Vyas S, Dixit A. Fatty acids/1-dodecanol binary eutectic phase change materials for low temperature solar thermal applications: Design, development and thermal analysis. Sol Energy. 2017;155:1373–9.
Konuklu Y, Paksoy HO, Unal M, Konuklu S. Microencapsulation of a fatty acid with Poly (melamine-urea-formaldehyde). Energ Convers Manage. 2014;80:382–90.
Huang JY, Lu SL, Kong XF, Liu SB, Li YR. Form-stable phase change materials based on eutectic mixture of tetradecanol and fatty acids for building energy storage: preparation and performance analysis. Materials. 2013;6(10):4758–75.
Yang XJ, Yuan YP, Zhang N, Cao XL, Liu C. Preparation and properties of myristic-palmitic-stearic acid/expanded graphite composites as phase change materials for energy storage. Sol Energy. 2014;99:259–66.
Liang K, Shi L, Zhang JY, Cheng J, Wang XD. Fabrication of shape-stable composite phase change materials based on lauric acid and graphene/graphene oxide complex aerogels for enhancement of thermal energy storage and electrical conduction. Thermochim Acta. 2018;664:1–15.
Sobolciak P, Karkri M, Al-Maaded MA, Krupa I. Thermal characterization of phase change materials based on linear low-density polyethylene, paraffin wax and expanded graphite. Renew Energ. 2016;88:372–82.
Zhang N, Yuan YP, Yuan YG, Cao XL, Yang XJ. Effect of carbon nanotubes on the thermal behavior of palmitic-stearic acid eutectic mixtures as phase change materials for energy storage. Sol Energy. 2014;110:64–70.
Han ZD, Fina A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: a review. Prog Polym Sci. 2011;36(7):914–44.
Xu BW, Li ZJ. Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailor-made for thermal energy storage cement-based composites. Energy. 2014;72:371–80.
Mitran RA, Berger D, Munteanu C, Matei C. Evaluation of different mesoporous silica supports for energy storage in shape-stabilized phase change materials with dual thermal responses. J Phys Chem C. 2015;119(27):15177–84.
Madani SH, Hu C, Silvestre-Albero A, Biggs MJ, Rodriguez-Reinoso F, Pendleton P. Pore size distributions derived from adsorption isotherms, immersion calorimetry, and isosteric heats: a comparative study. Carbon. 2016;96:1106–13.
Radhakrishnan R, Gubbins KE. Free energy studies of freezing in slit pores: an order-parameter approach using Monte Carlo simulation. Mol Phys. 1999;96(8):1249–67.
Li TX, Lee JH, Wang RZ, Kang YT. Enhancement of heat transfer for thermal energy storage application using stearic acid nanocomposite with multi-walled carbon nanotubes. Energy. 2013;55:752–61.
Acknowledgements
This work was financially supported by Guangdong Basic and Applied Basic Research Foundation (2020A1515011411), Key Research special Projects in Universities in Guangdong Province (2019KZDZX2002), the National Natural Science Foundation of China (31570572), and Guangzhou Science and Technology Project (201905010005) and the Project of Key Disciplines of Forestry Engineering of Bureau of Guangzhou Municipality.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, M., Guo, C. & Li, L. Preparation, characterization and thermal properties of dodecanol, palmitic acid and hydroxylpropyl methyl cellulose as novel form-stable phase change materials. J Therm Anal Calorim 147, 4915–4924 (2022). https://doi.org/10.1007/s10973-021-10915-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10915-y