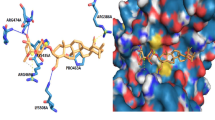
Abstract
P53 plays an important role in maintaining genetic stability and development of resistance against tumors. Dysregulation of P53 gene is one of the key factors contributing to the etiology of neuroblastoma which causes cells to evade apoptosis. Activating P53 pathway can be a therapeutic alternative to the currently available medicinal strategies. Mannich bases have been known to possess various biological activities including the anticancer activity. In this study, we have targeted the P53 pathway by novel Mannich base (3FB3FA8H) which can be a future prospect to cure neuroblastoma. 3FB3FA8H has shown modulation of P53 pathway leading to apoptosis of neuroblastoma cells. Mitochondrial membrane permeability is also increased by 3FB3FA8H which may be a consequence of P53 pathway modulation. 3FB3FA8H increases the mRNA levels of P53 leading to activation of BAX. Inclining BAX/BCL2 ratio towards apoptotic BAX leads to cleavage of caspase 3, ultimately, causing apoptosis. Series of experiments provide the evidence that Mannich base 3FB3FA8H leads to P53-mediated apoptosis. Inducing apoptosis by this mechanism could be of central importance in reducing tumor burden which can be a good prospect for neuroblastoma patients.







Similar content being viewed by others
References
Roman G (2015) Mannich bases in medicinal chemistry and drug design. Eur J Med Chem 89:743–816. https://doi.org/10.1016/j.ejmech.2014.10.076
Bala S, Sharma N, Kajal A, Kambo S, Saini V (2014) Mannich bases: an important pharmacophore in present scenario. Int J Med Chem 2014:191072. https://doi.org/10.1155/2014/191072
Mahal K, Ahmad A, Schmitt F, Lockhauserbäumer J, Starz K, Pradhan R, Padhye S, Sarkar FH, Koko WS, Schobert R, Ersfeld K, Biersack B (2017) Improved anticancer and antiparasitic activity of new lawsone Mannich bases. Eur J Med Chem 126:421–423. https://doi.org/10.1016/j.ejmech.2016.11.043
Gul M, Gul HI, Hänninen O (2002) Effects of Mannich bases on cellular glutathione and related enzymes of Jurkat cells in culture conditions. Toxicol In Vitro 16(2):107–112. https://doi.org/10.1016/s0887-2333(01)00115-1
Oliveri V, Vecchio G (2016) 8-Hydroxyquinoline in medicinal chemistry: a structural perspective. Eur J Med Chem 120:252–274. https://doi.org/10.1016/j.ejmech.2016.05.007
Shaw YA, Chang CY, Hsu MY, Lu PJ, Yang CN, Chen HL, Lo CW, Shiau CW, Chern MK (2010) Synthesis and structure-activity relationship study of 8-hydroxyquinoline-derived Mannich bases as anticancer agents. Eur J Med Chem 45:2860–2867. https://doi.org/10.1016/j.ejmech.2010.03.008
Andrew MD (2012) Neuroblastoma. Semin Pediatr Surg 21(1):2–14. https://doi.org/10.1053/j.sempedsurg.2011.10.009
Schwab M, Westermann F, Hero B, Berthold F (2003) Neuroblastoma: biology and molecular and chromosomal pathology. Lancet Oncol 4(8):472–480. https://doi.org/10.1016/s1470-2045(03)01166-5
Park JR, Eggert A, Caron H (2008) Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am 55(1):97–120. https://doi.org/10.1016/j.pcl.2007.10.014
Hou L, Ju C, Zhang J, Song J, Ge Y, Yue W (2008) Antitumor effects of Isatin on human neuroblastoma cell line (SH-SY5Y) and the related mechanism. Eur J Pharmacol 589(1):27–31. https://doi.org/10.1016/j.ejphar.2008.04.061
Castleberry RP (1997) Neuroblastoma. Eur J Cancer 33(9):1430–1437. https://doi.org/10.1016/s0959-8049(97)00308-0
American cancer Society (2017) Chemotherapy for neuroblastoma. https://www.cancer.org/cancer/neuroblastoma/treating/chemotherapy.html. Accessed 27 Jun 2017
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Lockshin RA, Zakeri Z (2007) Cell death in health and disease. J Cell Mol Med 11(6):1214–1224. https://doi.org/10.1111/j.1582-4934.2007.00150.x
Hannun YA (1997) Apoptosis and the dilemma of cancer chemotherapy. Blood 89(6):1845–1853
Gerschenson LE, Rotello RJ (1992) Apoptosis: a different type of cell death. FASEB J 6(7):2450–2455. https://doi.org/10.1096/fasebj.6.7.1563596
Kastan MB (2007) Wild-type p53: tumors can't stand it. Cell 128(5):837–840. https://doi.org/10.1016/j.cell.2007.02.022
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137(3):413–431. https://doi.org/10.1016/j.cell.2009.04.037
Ruiz-Perez VL et al (1999) ATP2A2 Mutations in Darier's disease: variant cutaneous phenotypes are associated with missense mutations, but neuropsychiatry features are independent of mutation class. Hum Mol Genet 8(9):1621–1630. https://doi.org/10.1093/hmg/8.9.1621
Laptenko O, Prives C (2006) Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ 13(6):951–961. https://doi.org/10.1038/sj.cdd.4401916
Kruse JP, Gu W (2009) Modes of p53 regulation. Cell 137(4):609–622. https://doi.org/10.1016/j.cell.2009.04.050
Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8(4):275–283. https://doi.org/10.1038/nrm2147
Balint E, Vousden KH (2001) Activation and activities of the p53 tumour suppressor protein. Br J Cancer 85(12):1813–1823. https://doi.org/10.1054/bjoc.2001.2128
Bouchet BP, de Fromentel CC, Puisieux A, Galmarini CM (2006) p53 as a target for anti-cancer drug development. Crit Rev Oncol Hematol 58(3):190–207. https://doi.org/10.1016/j.critrevonc.2005.10.005
Lewis JM, Truong TN, Schwartz MA (2002) Integrins regulate the apoptotic response to DNA damage through modulation of p53. Proc Natl Acad Sci USA 99(6):3627–3632. https://doi.org/10.1073/pnas.062698499
Rich T, Allen RL, Wyllie AH (2000) Defying death after DNA damage. Nature 407(6805):777–783. https://doi.org/10.1038/35037717
Burns TF, El-Deiry WS (1999) The p53 pathway and apoptosis. J Cell Physiol 181(2):231–239. https://doi.org/10.1002/(sici)1097-4652(199911)181:2%3C231:aid-jcp5%3E3.0.co;2-l
Haupt S, Berger M, Goldberg Z, Haupt Y (2003) Apoptosis-the p53 network. J Cell Sci 116(20):4077–4085. https://doi.org/10.1242/jcs.00739
Toshiyuki M, Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80(2):293–299. https://doi.org/10.1016/0092-8674(95)90412-3
Li R, Hannon GJ, Beach D, Stillman B (1996) Subcellular distribution of p21 and PCNA in normal and repair-deficient cells following DNA damage. Curr Biol 6(2):189–199. https://doi.org/10.1016/s0960-9822(02)00452-9
Hansen MB, Nielsen SE, Berg K (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119(2):203–210. https://doi.org/10.1016/0022-1759(89)90397-9
Hanif F, Perveen K, Jawed H, Ahmed A, Malhi SM, Jamall S, Simjee SU (2014) N-(2-Hydroxyphenyl) acetamide (NA-2) and temozolomide synergistically induce apoptosis in human glioblastoma cell line U87. Cancer Cell Int 14(1):133. https://doi.org/10.1186/s12935-014-0133-5
DeCoster MA (2007) The nuclear factor (NAF): a measure for cell apoptosis using microscopy and image analysis. In: Modern research and educational topics in microscopy, pp 378–384
Susin SA et al (2000) Two distinct pathways leading to nuclear apoptosis. J Exp Med 192(4):571–580. https://doi.org/10.1084/jem.192.4.571
Bron D, Mark A (2004) Quantification of sPLA2-induced early and late apoptosis changes in neuronal cell cultures using combined TUNEL and DAPI staining. Brain Res Protoc 13:144–150. https://doi.org/10.1016/j.brainresprot.2004.04.001
Li PF, Dietz R, Von Harsdorf R (1999) p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J 18(21):6027–6036. https://doi.org/10.1093/emboj/18.21.6027
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257. https://doi.org/10.1038/bjc.1972.33
Faizi S, Saleem R, Siddiqui BS, Gilani SS et al (1993) Mannich reaction of 8-hydroxyquinoline (oxine): potential antifungal and analgesic agents. Proc Pak Acad Sci 30(4):231–246
Zhou Y, Wang J, Gu Z, Wang S, Zhu W, Acena JL, Soloshonok VA, Izawa K, Liu H (2016) Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem Rev 116:422–518. https://doi.org/10.1021/acs.chemrev.5b00392
Pati HN, Das U, Sharma RK, Dimmock JR (2007) Cytotoxic thiol alkylators. Mini-Rev. Med Chem 7(2):131–139. https://doi.org/10.2174/138955707779802642
Rainwater R, Parks D, Anderson ME, Tegtmeyer P, Mann K (1995) Role of cysteine residues in regulation of p53 function. Mol Cell Biol 15(7):3892–3903. https://doi.org/10.1128/mcb.15.7.3892
Giaccia AJ, Kastan MB (1998) The complexity of P53 modulation: emerging patterns from divergent signals. Genes Dev 12:2973–2983. https://doi.org/10.1101/gad.12.19.2973
Lohrum MA, Vousden KH (1999) Regulation and activation of P53 and its family members. Cell Death Differ 6:1162–1168. https://doi.org/10.1038/sj.cdd.4400625
Ko LJ, Prives C (1996) p53: puzzle and paradigm. Genes Dev 10(9):1054–1072. https://doi.org/10.1101/gad.10.9.1054
Liontas A, Yeger H (2004) Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res 24(2B):987–998
Nikolaev AY, Li M, Puskas N, Qin J, Gu W (2003) Parc: a cytoplasmic anchor for p53. Cell 112(1):29–40. https://doi.org/10.1016/s0092-8674(02)01255-2
Kobayashi T, Sawa H, Morikawa J, Zhang W, Shiku H (2000) Bax induction activates apoptotic cascade via mitochondrial cytochrome c release and Bax overexpression enhances apoptosis induced by chemotherapeutic agents in DLD-1 colon cancer cells. Cancer Sci 91(12):1264–1268. https://doi.org/10.1111/j.1349-7006.2000.tb00913.x
Oltval ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 74(4):609–619. https://doi.org/10.1016/0092-8674(93)90509-o
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408(6810):307–310. https://doi.org/10.1038/35042675
Moran LK, Gutteridge J, Quinlan GJ (2001) Thiols in cellular redox signalling and control. Curr Med Chem 8(7):763–772. https://doi.org/10.2174/0929867013372904
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12(5):835–840. https://doi.org/10.1007/s10495-006-0525-7
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM (2012) p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149(7):1536–1548. https://doi.org/10.1016/j.cell.2012.05.014
Cui H, Schroering A, Ding HF (2002) Mol Cancer Ther 1(9):679–686
Laverdière C, Liu Q, Yasui Y, Nathan PC, Gurney JG, Stovall M, Sklar CA (2009) Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 101(16):1131–1140. https://doi.org/10.1093/jnci/djp230
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussain, S.S., Faizi, S., Rafi, K. et al. Novel Mannich base 3FB3FA8H induces apoptosis by upregulating P53 pathway in neuroblastoma cells. Mol Cell Biochem 471, 29–39 (2020). https://doi.org/10.1007/s11010-020-03755-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03755-1