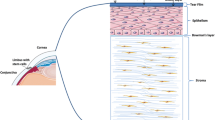
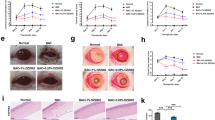
Abstract
Autophagy is an intracellular catabolic process implicated in the recycling and degradation of intracellular components. Few studies have defined its role in corneal pathologies. Animal models are essential for understanding autophagy regulation and identifying new treatments to modulate its effects. A systematic review (SR) was conducted of studies employing animal models for investigations of autophagy in corneal diseases. Studies were identified using a structured search strategy (TS = autophagy AND cornea*) in Web of Science, Scopus, and PubMed from inception to September 2019. In this study, 230 articles were collected, of which 28 were analyzed. Mouse models were used in 82% of the studies, while rat, rabbit, and newt models were used in the other 18%. The most studied corneal layer was the epithelium, followed by the endothelium and stroma. In 13 articles, genetically modified animal models were used to study Fuch endothelial corneal dystrophy (FECD), granular corneal dystrophy type 2 (GCD2), dry eye disease (DED), and corneal infection. In other 13 articles, animal models were experimentally induced to mimic DED, keratitis, inflammation, and surgical scenarios. Furthermore, in 50% of studies, modulators that activated or inhibited autophagy were also investigated. Protective effects of autophagy activators were demonstrated, including rapamycin for DED and keratitis, lithium for FECD, LYN-1604 for DED, cysteamine and miR-34c antagomir for damaged corneal epithelium. Three autophagy suppressors were also found to have therapeutic effects, such as aminoimidazole-4-carboxamide-riboside (AICAR) for corneal allogeneic transplantation, celecoxib and chloroquine for DED.


Similar content being viewed by others
Abbreviations
- AICAR:
-
Aminoimidazole-4-carboxamide riboside
- AMD:
-
Age-related macular degeneration
- AMPK:
-
AMP-activated protein kinase
- ATGs:
-
Autophagy-related
- BBD:
-
Beclin-binding domain
- BECN1:
-
Beclin-1
- COX:
-
Cyclooxygenase
- CsA:
-
Cyclosporine A
- DC:
-
Dendritic cells
- DED:
-
Dry eye disease
- DDIT4:
-
DNA damage-inducible transcript 4
- ECM:
-
Extracellular matrix
- ELISA:
-
Enzyme-linked immunosorbent assay
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal regulated kinase
- FECD:
-
Fuch endothelial corneal dystrophy
- FoxO3:
-
Forkhead box O3
- FYCO1:
-
Coiled-coil domain containing 1
- GCD2:
-
Granular corneal dystrophy type 2
- GFAP:
-
Glial fibrillary acidic protein
- H&E:
-
Hematoxylin–eosin
- HCEC:
-
Human corneal epithelial cells
- HIF-1α:
-
Hypoxia-inducible factor
- HSK:
-
Herpetic stromal keratitis
- HSV-1:
-
Herpes simplex virus
- ICNs:
-
Intraepithelial corneal nerves
- IFNγ:
-
Gamma interferon
- IL:
-
Interleukin
- IMPase:
-
Inositol monophosphatase
- INT:
-
Intraepithelial nerve terminal
- IRF3:
-
IFN regulatory factor 3
- LAMP:
-
Lysosomal-associated membrane protein
- LYVE-1:
-
Lymphatic vascular endothelial gene
- MAP1LC3:
-
Microtubule-associated protein 1 light chain 3
- MHCII:
-
Histocompatibility complex class II
- MMC:
-
Mitomycin-C
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin complex
- NAC:
-
N-acetylcysteine
- NO:
-
Nitric oxide
- NOD:
-
Non-obese diabetic
- NOX4:
-
Nicotinamide adenine dinucleotide phosphate oxidase 4
- MMP:
-
Matrix metalloproteinase
- PAS:
-
Periodic acid Schiff
- PDGFR:
-
Platelet-derived growth factor receptors
- PECAM:
-
Platelet endothelial cell adhesion molecule
- PI3KC3, also known as VPS34:
-
Phosphatidylinositol 3-kinase, catalytic subunit type 3
- PI3P:
-
Phosphatidylinositol 3-phosphate
- PI3K:
-
Phosphatidylinositol-3-kinase
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analysis
- PROSPERO:
-
Prospective Register of Systematic Reviews
- PrP:
-
Protein prion
- PUMA:
-
P53 upregulated modulator of apoptosis
- qPCR:
-
Quantitative polymerase chain reaction
- ROCK:
-
Rho-associated protein kinase
- ROS:
-
Reactive oxygen species
- RPE:
-
Retinal pigmented epithelium
- SiNPs:
-
Nonporous silica nanoparticles
- Sirt3:
-
Silent mating type information regulation 2 homolog 3
- SR:
-
Systematic review
- SYRCLE’s:
-
Systematic Review Center for Laboratory Animal Experimentation’s
- TFEB:
-
Transcription factor EB
- TGFB1:
-
Transforming growth factor beta 1
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- TNF:
-
Tumor necrosis factor
- TSC:
-
Tuberous Sclerosis Complex
- ULK1:
-
UNC-51-like kinase
- UPR:
-
Unfolded protein response
- UPS:
-
Ubiquitin–proteasome system
- VEGF:
-
Vascular endothelial growth factor
- 3-MA:
-
3-Methyladenine
References
González-Polo RA, Pizarro-Estrella E, Yakhine-Diop SMS et al (2015) Is the modulation of autophagy the future in the treatment of neurodegenerative diseases? Curr Top Med Chem 15:2152–2174
Tanida I (2011) Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal 14:2201–2214. https://doi.org/10.1089/ars.2010.3482
Yakhine-Diop SMS, Martínez-Chacón G, González-Polo RA et al (2017) Fluorescent FYVE chimeras to quantify PtdIns3P synthesis during autophagy. Methods Enzymol 587:257–269. https://doi.org/10.1016/bs.mie.2016.09.060
Rubinsztein DC, Shpilka T, Elazar Z (2012) Mechanisms of autophagosome biogenesis. Curr Biol 22:R29–R34. https://doi.org/10.1016/j.cub.2011.11.034
Bjørkøy G, Lamark T, Pankiv S et al (2009) Chapter 12 monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 451:181–197. https://doi.org/10.1016/S0076-6879(08)03612-4
Kim YC, Guan K, Kim YC, Guan K (2015) mTOR: a pharmacologic target for autophagy regulation. 125:25–32. https://doi.org/10.1172/JCI73939.such
Bukowiecki A, Hos D, Cursiefen C, Eming SA (2017) Wound-healing studies in cornea and skin: parallels, differences and opportunities. Int J Mol Sci 18:1–24. https://doi.org/10.3390/ijms18061257
Bron AJ (2001) The architecture of the corneal stroma. Br J Ophthalmol 85:379–381. https://doi.org/10.1136/bjo.85.4.379
Dua HS, Faraj LA, Said DG et al (2013) Human corneal anatomy redefined: a novel pre-descemet’s layer (Dua’s layer). Ophthalmology 120:1778–1785. https://doi.org/10.1016/j.ophtha.2013.01.018
Moshirfar M, Murri MS, Shah TJ et al (2019) A review of corneal endotheliitis and endotheliopathy: differential diagnosis, evaluation, and treatment. Ophthalmol Ther 8:195–213. https://doi.org/10.1007/s40123-019-0169-7
Yee RW, Matsuda M, Schultz RO, Edelhauser HF (1985) Changes in the normal corneal endothelial cellular pattern as a function of age. Curr Eye Res 4:671–678. https://doi.org/10.3109/02713688509017661
Boya P, Esteban-Martínez L, Serrano-Puebla A et al (2016) Autophagy in the eye: development, degeneration, and aging. Prog Retin Eye Res 55:206–245. https://doi.org/10.1016/j.preteyeres.2016.08.001
Robaei D, Watson S (2014) Corneal blindness: a global problem. Clin Exp Ophthalmol 42:213–214. https://doi.org/10.1111/ceo.12330
Martin LM, Jeyabalan N, Tripathi R et al (2019) Autophagy in corneal health and disease: a concise review. Ocul Surf 17:186–197. https://doi.org/10.1016/j.jtos.2019.01.008
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. https://doi.org/10.1038/nature06639
Chai P, Ni H, Zhang H, Fan X (2016) The evolving functions of autophagy in ocular health: a double-edged sword. Int J Biol Sci 12:1332–1340. https://doi.org/10.7150/ijbs.16245
Xie Z, Klionsky DJ (2007) Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9:1102–1109. https://doi.org/10.1038/ncb1007-1102
Smith JA, Al-beitz J, Begley C, Caffery B, Nichols K, Schaumberg D, Schein O (2007) The epidemiology of dry eye disease: report of the epidemiology subcommitee of The International Dry Eye WorkShop. Ocul Surf 5:93–107. https://doi.org/10.1016/S1542-0124(12)70082-4
Ogawa Y, Tsubota K (2013) Dry eye disease and inflammation. Inflamm Regen 33:238–248. https://doi.org/10.2492/inflammregen.33.238
Kim EC, Meng H, Jun AS (2013) Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br J Ophthalmol 97:1068–1073. https://doi.org/10.1136/bjophthalmol-2012-302881
Meng H, Matthaei M, Ramanan N et al (2013) L450W and Q455K Col8a2 knock-in mouse models of fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Investig Ophthalmol Vis Sci 54:1887–1897. https://doi.org/10.1167/iovs.12-11021
Petrovski G, Pásztor K, Orosz L et al (2014) Herpes simplex virus types 1 and 2 modulate autophagy in SIRC corneal cells. J Biosci 39:683–692. https://doi.org/10.1007/s12038-014-9443-y
Reme CE, Young RW (1977) The effects of hibernation on cone visual cells in the ground squirrel. Investig Ophthalmol Vis Sci 16:815–840
Shetty R, Sharma A, Pahuja N et al (2017) Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. PLoS ONE 12:e0184628. https://doi.org/10.1371/journal.pone.0184628
Choi S-I, Kim B-Y, Dadakhujaev S et al (2012) Impaired autophagy and delayed autophagic clearance of transforming growth factor β-induced protein (TGFBI) in granular corneal dystrophy type 2. Autophagy 8:1782–1797. https://doi.org/10.4161/auto.22067
Szabó DJ, Nagymihály R, Veréb Z, et al (2018) Ex vivo 3D human corneal stroma model for {Schnyder} corneal dystrophy—role of autophagy in its pathogenesis and resolution. Histol Histopathol 33:455–462. https://doi.org/10.14670/HH-11-928
Il CS, Kim KS, Oh JY et al (2013) Melatonin induces autophagy via an mTOR-dependent pathway and enhances clearance of mutant-TGFBIp. J Pineal Res 54:361–372. https://doi.org/10.1111/jpi.12039
Nie D, Peng Y, Li M et al (2018) Lithium chloride (LiCl) induced autophagy and downregulated expression of transforming growth factor β-induced protein (TGFBI) in granular corneal dystrophy. Exp Eye Res 173:44–50. https://doi.org/10.1016/j.exer.2018.04.008
Vakifahmetoglu-norberg H, Xia H, Vakifahmetoglu-norberg H et al (2015) Pharmacologic agents targeting autophagy Find the latest version: pharmacologic agents targeting autophagy. J Clin Invest 125:5–13. https://doi.org/10.1172/JCI73937.conditionally
Klionsky DJ, Abdelmohsen K, Abe A et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222. https://doi.org/10.1080/15548627.2015.1100356
McWilliams TG, Prescott AR, Villarejo-Zori B et al (2019) A comparative map of macroautophagy and mitophagy in the vertebrate eye. Autophagy 15:1296–1308. https://doi.org/10.1080/15548627.2019.1580509
Neumann S, Romonath R (2009) Faculty of human sciences pedagogics & therapy of speech and language disorders effectiveness of nasopharyngoscopic biofeedback in clients with cleft palate speech—a systematic review. PLoS Med 6:50931. https://doi.org/10.1371/journal.pmed.1000100
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. https://doi.org/10.1371/journal.pmed.1000100
Hooijmans CR, Rovers MM, De Vries RBM et al (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:1–9. https://doi.org/10.1186/1471-2288-14-43
Wang B, Peng L, Ouyang H et al (2019) Induction of DDIT4 impairs autophagy through oxidative stress in dry eye. Invest Ophthalmol Vis Sci 60(8):2836–2847
Wang G, Xue Y, Wang Y et al (2019) The role of autophagy in the pathogenesis of exposure keratitis. J Cell Mol Med 23:4217–4228. https://doi.org/10.1111/jcmm.14310
He J, Pham TL, Kakazu AH, Bazan HEP (2019) Remodeling of substance P sensory nerves and transient receptor potential melastatin 8 (TRPM8) cold receptors after corneal experimental surgery. Investig Ophthalmol Vis Sci 60:2449–2460. https://doi.org/10.1167/iovs.18-26384
Hu J, Hu X, Kan T (2019) MiR-34c participates in diabetic corneal neuropathy via regulation of autophagy. Investig Ophthalmol Vis Sci 60:16–25. https://doi.org/10.1167/iovs.18-24968
Hu J, Kan T, Hu X (2019) Sirt3 regulates mitophagy level to promote diabetic corneal epithelial wound healing. Exp Eye Res 181:223–231. https://doi.org/10.1016/j.exer.2019.02.011
Korom M, Wylie KM, Wang H et al (2013) A proautophagic antiviral role for the cellular prion protein identified by infection with a herpes simplex virus 1 ICP34.5 mutant. J Virol 87:5882–5894. https://doi.org/10.1128/JVI.02559-12
Lee HJ, Shin S, Yoon S-G et al (2019) The effect of chloroquine on the development of dry eye in Sjögren Syndrome animal model. Investig Opthalmology Vis Sci 60:3708. https://doi.org/10.1167/iovs.19-27469
Leib DA, Alexander DE, Cox D et al (2009) Interaction of ICP34.5 with beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol 83:12164–12171. https://doi.org/10.1128/JVI.01676-09
Jiang L, Liu T, Xie L et al (2019) AICAR prolongs corneal allograft survival via the AMPK-mTOR signaling pathway in mice. Biomed Pharmacother 113:108558. https://doi.org/10.1016/j.biopha.2019.01.019
Ma S, Yu Z, Feng S et al (2019) Corneal autophagy and ocular surface inflammation: a new perspective in dry eye. Exp Eye Res 184:126–134. https://doi.org/10.1016/j.exer.2019.04.023
Miao Q, Xu Y, Zhang H et al (2019) Cigarette smoke induces ROS mediated autophagy impairment in human corneal epithelial cells. Environ Pollut (Barking, Essex, 1987) 245:389–397. https://doi.org/10.1016/j.envpol.2018.11.028
Parker ZM, Murphy AA, Leib DA (2015) Role of the DNA Sensor STING in protection from lethal infection following corneal and intracerebral challenge with herpes simplex virus 1. J Virol 89:11080–11091. https://doi.org/10.1128/jvi.00954-15
Seo Y, Ji YW, Lee SM et al (2014) Activation of HIF-1α (hypoxia inducible factor-1α) prevents dry eye-induced acinar cell death in the lacrimal gland. Cell Death Dis 5:e1309. https://doi.org/10.1038/cddis.2014.260
Shah M, Edman MC, Reddy Janga S et al (2017) Rapamycin eye drops suppress lacrimal gland inflammation in a murine model of Sjögren’s Syndrome. Invest Ophthalmol Vis Sci 58:372–385. https://doi.org/10.1167/iovs.16-19159
Stepp MA, Pal-Ghosh S, Tadvalkar G et al (2018) Reduced intraepithelial corneal nerve density and sensitivity accompany desiccating stress and aging in C57BL/6 mice. Exp Eye Res 169:91–98. https://doi.org/10.1016/j.exer.2018.01.024
Stepp MA, Pal-Ghosh S, Tadvalkar G et al (2018) Reduced corneal innervation in the CD25 null model of Sjögren syndrome. Int J Mol Sci. https://doi.org/10.3390/ijms19123821
Yamazoe K, Yoshida S, Yasuda M et al (2015) Development of a transgenic mouse with R124H human TGFBI mutation associated with granular corneal dystrophy type 2. PLoS ONE 10:e0133397. https://doi.org/10.1371/journal.pone.0133397
Jiang Y, Yin X, Stuart PM, Leib DA (2015) Dendritic cell autophagy contributes to herpes simplex virus-driven stromal keratitis and immunopathology. MBio. https://doi.org/10.1128/mBio.01426-15
Zhang F, Li Y, Tang Z et al (2012) Proliferative and survival effects of PUMA promote angiogenesis. Cell Rep 2:1272–1285. https://doi.org/10.1016/j.celrep.2012.09.023
Li C, Li C, Li H, et al (2019) Disparate expression of autophagy in corneas of C57BL/6 mice and BALB/c mice after Aspergillus fumigatus infection. Int J Ophthalmol 12:705–710. https://doi.org/10.18240/ijo.2019.05.02
Uchida K, Unuma K, Funakoshi T et al (2014) Activation of master autophagy regulator TFEB during systemic LPS administration in the cornea. J Toxicol Pathol 27:153–158. https://doi.org/10.1293/tox.2014-0004
Yin Y, Zong R, Bao X et al (2018) Oxidative stress suppresses cellular autophagy in corneal epithelium. Invest Ophthalmol Vis Sci 59:3286–3293. https://doi.org/10.1167/iovs.18-24057
Di Tommaso C, Torriglia A, Furrer P et al (2011) Ocular biocompatibility of novel Cyclosporin A formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int J Pharm 416:515–524. https://doi.org/10.1016/j.ijpharm.2011.01.004
Kim J-Y, Park J-H, Kim M et al (2017) Safety of nonporous silica nanoparticles in human corneal endothelial cells. Sci Rep 7:14566. https://doi.org/10.1038/s41598-017-15247-2
Kanao T, Miyachi Y (2006) Lymphangiogenesis promotes lens destruction and subsequent lens regeneration in the newt eyeball, and both processes can be accelerated by transplantation of dendritic cells. Dev Biol 290:118–124. https://doi.org/10.1016/j.ydbio.2005.11.017
Kim HS, Il CS, Jeung EB, Yoo YM (2014) Cyclosporine A induces apoptotic and autophagic cell death in rat pituitary GH3 cells. PLoS ONE 9:1–9. https://doi.org/10.1371/journal.pone.0108981
Y. Zernii E, E. Baksheeva V, N. Iomdina E, et al (2016) Rabbit models of ocular diseases: new relevance for classical approaches. CNS Neurol Disord Drug Targets 15:267–291. https://doi.org/10.2174/1871527315666151110124957
Koizumi N, Okumura N, Kinoshita S (2012) Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res 95:60–67. https://doi.org/10.1016/j.exer.2011.10.014
Kimura T, Jain A, Choi SW et al (2017) TRIM-directed selective autophagy regulates immune activation. Autophagy 13:989–990. https://doi.org/10.1080/15548627.2016.1154254
Yang J, Chen D, He Y et al (2013) MiR-34 modulates Caenorhabditis elegans lifespan via repressing the autophagy gene atg9. Age (Omaha) 35:11–22. https://doi.org/10.1007/s11357-011-9324-3
Yakhine-Diop SMS, Rodríguez-Arribas M, Martínez-Chacón G et al (2018) Acetylome in human fibroblasts from Parkinson’s disease patients. Front Cell Neurosci 12:1–8. https://doi.org/10.3389/fncel.2018.00097
Aldrich BT, Schlötzer-Schrehardt U, Skeie JM et al (2017) Mitochondrial and morphologic alterations in native human corneal endothelial cells associated with diabetes mellitus. Investig Ophthalmol Vis Sci 58:2130–2138. https://doi.org/10.1167/iovs.16-21094
Chen J, Ma Z, Jiao X et al (2011) Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet 88:827–838. https://doi.org/10.1016/j.ajhg.2011.05.008
Morishita H, Eguchi S, Kimura H et al (2013) Deletion of autophagy-related 5 (Atg5) and Pik3c3 genes in the lens causes cataract independent of programmed organelle degradation. J Biol Chem 288:11436–11447. https://doi.org/10.1074/jbc.M112.437103
Rezaie T, Child A, Hitchings R et al (2002) Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295:1077–1079. https://doi.org/10.1126/science.1066901
Mitter SK, Song C, Qi X et al (2014) Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 10:1989–2005. https://doi.org/10.4161/auto.36184
Park HYL, Kim JH, Park CK (2012) Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis 3:e290–e299. https://doi.org/10.1038/cddis.2012.26
Zhao C, Yasumura D, Li X et al (2011) mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest 121:369–383. https://doi.org/10.1172/JCI44303
Yao J, Tao ZF, Li CP et al (2014) Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell Physiol Biochem 33:107–116. https://doi.org/10.1159/000356654
Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G et al (2016) Topical Mitomycin-C enhances subbasal nerve regeneration and reduces erosion frequency in the debridement wounded mouse cornea. Exp Eye Res 146:361–369. https://doi.org/10.1016/j.exer.2015.08.023
Schmidl D, Werkmeister R, Kaya S et al (2017) A controlled, randomized double-blind study to evaluate the safety and efficacy of chitosan-N-acetylcysteine for the treatment of dry eye syndrome. J Ocul Pharmacol Ther 33:375–382. https://doi.org/10.1089/jop.2016.0123
Matsuo T, Tsuchida Y, Morimoto N (2002) Trehalose eye drops in the treatment of dry eye syndrome. Ophthalmology 109:2024–2029. https://doi.org/10.1016/S0161-6420(02)01219-8
Lu Y, Liu LL, Liu SS et al (2016) Celecoxib suppresses autophagy and enhances cytotoxicity of imatinib in imatinib-resistant chronic myeloid leukemia cells. J Transl Med 14:1–13. https://doi.org/10.1186/s12967-016-1012-8
Zhang L, Fu L, Zhang S et al (2017) Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chem Sci 8:2687–2701. https://doi.org/10.1039/C6SC05368H
Moscovici BK, Holzchuh R, Sakassegawa-Naves FE et al (2015) Treatment of Sjögren’s syndrome dry eye using 0.03% tacrolimus eye drop: prospective double-blind randomized study. Contact Lens Anterior Eye 38:373–378. https://doi.org/10.1016/j.clae.2015.04.004
Koch JC, Tatenhorst L, Roser AE et al (2018) ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol Ther 189:1–21. https://doi.org/10.1016/j.pharmthera.2018.03.008
Park JH, Kim JY, Kim DJ et al (2017) Effect of nitric oxide on human corneal epithelial cell viability and corneal wound healing. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-08576-9
Sarkar S, Korolchuk VI, Renna M et al (2011) Complex inhibitory effects of nitric oxide on autophagy. Mol Cell 43:19–32. https://doi.org/10.1016/j.molcel.2011.04.029
De Stefano D, Villella VR, Esposito S et al (2014) Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy 10:2053–2074. https://doi.org/10.4161/15548627.2014.973737
Sarkar S, Floto RA, Berger Z et al (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170:1101–1111. https://doi.org/10.1083/jcb.200504035
Hall DT, Griss T, Ma JF, et al (2018) Inflammation-associated cachectic muscle wasting. EMBO Mol Med. https://doi.org/10.15252/emmm.201708307
Shivakumar S, Panigrahi T, Shetty R et al (2018) Chloroquine protects human corneal epithelial cells from desiccation stress induced inflammation without altering the autophagy flux. Biomed Res Int. https://doi.org/10.1155/2018/7627329
Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B (2013) Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine A-induced cell death. Oncogene 32:1518–1529. https://doi.org/10.1038/onc.2012.174
Okumura N, Kinoshita S, Koizumi N (2017) Application of Rho kinase inhibitors for the treatment of corneal endothelial diseases. J Ophthalmol. https://doi.org/10.1155/2017/2646904
Linares-Alba MA, Gómez-Guajardo MB, Fonzar JF et al (2016) Preformulation studies of a liposomal formulation containing sirolimus for the treatment of dry eye disease. J Ocul Pharmacol Ther 32:11–22. https://doi.org/10.1089/jop.2015.0032
El Sanharawi M, Kowalczuk L, Touchard E et al (2010) Protein delivery for retinal diseases: from basic considerations to clinical applications. Prog Retin Eye Res 29:443–465. https://doi.org/10.1016/j.preteyeres.2010.04.001
Okumura N, Koizumi N, Ueno M et al (2012) ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol 181:268–277. https://doi.org/10.1016/j.ajpath.2012.03.033
Acknowledgements
The systematic review was performed at the Jesús Usón Minimally Invasive Surgery Center (CCMIJU) which is part of the ICTS “Nanbiosis.” G.MC was supported by ONCE Foundation. F.J. Vela, J.L. Campos, E. Abellán and A. Ballestín were supported by Jesús Usón Minimally Invasive Surgery Foundation. S.M.S. Y-D was supported by Isabel Gemio Foundation. Authors thank Raquel Lozano Delgado for the illustration of the Fig. 2. Autophagy modulators targeting different steps of the autophagic machinery in corneal diseases, and FUNDESALUD for helpful assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martínez-Chacón, G., Vela, F.J., Campos, J.L. et al. Autophagy modulation in animal models of corneal diseases: a systematic review. Mol Cell Biochem 474, 41–55 (2020). https://doi.org/10.1007/s11010-020-03832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03832-5