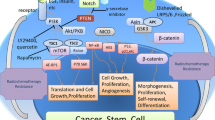
Abstract
With ever increasing evidences on the role of fusion genes as the oncogenic protagonists in myriad cancers, it’s time to explore if fusion genes can be the next generational drug targets in meeting the current demands of higher drug efficacy. Eliminating cancer stem cells (CSC) has become the current focus; however, we have reached a standstill in drug development owing to the lack of effective strategies to eradicate CSC. We believe that fusion genes could be the novel targets to overcome this limitation. The intriguing feature of fusion genes is that it dominantly impacts every aspect of CSC including self-renewal, differentiation, lineage commitment, tumorigenicity and stemness. Given the clinical success of fusion gene-based drugs in hematological cancers, our attempt to target fusion genes in eradicating CSC can be rewarding. As fusion genes are expressed explicitly in cancer cells, eradicating CSC by targeting fusion genes provides yet an another advantage of negligible patient side effects since normal cells remain unaffected by the drug. We hereby delineate the latest evidences on how fusion genes regulate CSC and drug resistance.




Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not Applicable.
References
Phi LTH et al (2018) Cancer Stem Cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells International 2018:1–16. https://doi.org/10.1155/2018/5416923
Kim S-C et al (2019) Identification of a novel fusion gene, FAM174A-WWC1, in early-onset colorectal cancer: establishment and characterization of four human cancer cell lines from early-onset colorectal cancers. Translational Oncology 12(9):1185–1195. https://doi.org/10.1016/j.tranon.2019.05.019
Dai X, Theobard R, Cheng H, Xing M, Zhang J (2018) Fusion genes: A promising tool combating against cancer. Biochim Biophys Acta Rev Cancer. https://doi.org/10.1016/j.bbcan.2017.12.003
Shaw AT et al (2013) Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1214886
Iqbal N, Iqbal N (2014) Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract. https://doi.org/10.1155/2014/357027
Doebele RC et al (2020) Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. https://doi.org/10.1016/S1470-2045(19)30691-6
Cho Y, Kim YK (2020) Cancer stem cells as a potential target to overcome multidrug resistance. Front Oncol. https://doi.org/10.3389/fonc.2020.00764
Moore N, Lyle S (2010) Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol. https://doi.org/10.1155/2011/396076
Morodomi Y et al (2014) Non-small cell lung cancer patients with EML4-ALK fusion gene are insensitive to cytotoxic chemotherapy. Anticancer Res 34:3825–3830
Xie J, Gu D, Song R (2018) Abstract 897: A novel fusion gene responsible for colon cancer drug resistance. Cancer Res. https://doi.org/10.1158/1538-7445.AM2018-897
Slupianek A et al (2002) Fusion tyrosine kinases induce drug resistance by stimulation of homology-dependent recombination repair, prolongation of G2/M phase, and protection from apoptosis. Mol Cell Biol. https://doi.org/10.1128/MCB.22.12.4189-4201.2002
Xu XF et al (2019) BMX-ARHGAP fusion protein maintains the tumorigenicity of gastric cancer stem cells by activating the JAK/STAT3 signaling pathway. Cancer Cell Int. https://doi.org/10.1186/s12935-019-0847-5
Zhou Y et al (2017) Evaluation of expression of cancer stem cell markers and fusion gene in synovial sarcoma: Insights into histogenesis and pathogenesis. Oncol Rep. https://doi.org/10.3892/or.2017.5617
Zhong Q et al (2018) The RARS–MAD1L1 fusion gene induces cancer stem cell–like properties and therapeutic resistance in nasopharyngeal carcinoma. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-17-0352
Riggi N et al (2008) EWS-FLI-1 expression triggers a Ewing’s sarcoma initiation program in primary human mesenchymal stem cells. Can Res. https://doi.org/10.1158/0008-5472.CAN-07-1761
Parker BC, Zhang W (2013) Fusion genes in solid tumors: an emerging target for cancer diagnosis and treatment. Chin J Cancer. https://doi.org/10.5732/cjc.013.10178
Kreso A, Dick JE (2014) Evolution of the cancer stem cell model. Cell Stem Cell. https://doi.org/10.1016/j.stem.2014.02.006
Afify S, Seno M (2019) Conversion of stem cells to cancer stem cells: Undercurrent of cancer initiation. Cancers. https://doi.org/10.3390/cancers11030345
Brücher BLDM, Jamall IS (2016) Somatic mutation theory - why it’s wrong for most cancers. Cell Physiol Biochem. https://doi.org/10.1159/000443106
Capp JP (2019) Cancer stem cells: From historical roots to a new perspective. Journal of Oncology. https://doi.org/10.1155/2019/5189232
Bighetti-Trevisan RL, Sousa LO, Castilho RM, Almeida LO (2019) Cancer stem cells: powerful targets to improve current anticancer therapeutics. Stem Cells International. https://doi.org/10.1155/2019/9618065
Begicevic R-R, Falasca M (2017) ABC transporters in cancer stem cells: beyond chemoresistance. Int J Mol Sci. https://doi.org/10.3390/ijms18112362
Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R (2014) Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1324297111
Jaiswal S, Traver D, Miyamoto T, Akashi K, Lagasse E, Weissman IL (2003) Expression of BCR/ABL and BCL-2 in myeloid progenitors leads to myeloid leukemias. Proc Natl Acad Sci 100(17):10002–10007. https://doi.org/10.1073/pnas.1633833100
Lagasse E (2007) Cancer stem cells with genetic instability: the best vehicle with the best engine for cancer. Gene Therapy. https://doi.org/10.1038/sj.gt.3303068
Chávez-González A, Avilés-Vázquez S, Moreno-Lorenzana D, Mayani H (2013) Hematopoietic stem cells in chronic myeloid leukemia. Stem cell biology in normal life and diseases. https://doi.org/10.5772/54651
Affer M et al (2011) Gene Expression Differences between Enriched Normal and Chronic Myelogenous Leukemia Quiescent stem/progenitor cells and correlations with biological abnormalities. Journal of Oncology 1–25:2011. https://doi.org/10.1155/2011/798592
Rowley JD (1998) The critical role of chromosome translocations in human leukemias. Annu Rev Genet 32:495–519. https://doi.org/10.1146/annurev.genet.32.1.495
Ayton PM, Cleary ML (2003) Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev 17:2298–2307. https://doi.org/10.1101/gad.1111603
Ayton PM, Cleary ML (2001) Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene. https://doi.org/10.1038/sj.onc.1204639
Chopra M, Bohlander SK (2019) “The cell of origin and the leukemia stem cell in acute myeloid leukemia”, Genes. Chromosomes and Cancer. https://doi.org/10.1002/gcc.22805
Cozzio A (2003) Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. https://doi.org/10.1101/gad.1143403
Schneidawind C et al (2018) MLL leukemia induction by t(9;11) chromosomal translocation in human hematopoietic stem cells using genome editing. Blood Adv. https://doi.org/10.1182/bloodadvances.2017013748
Stavropoulou V, Peters AHFM, Schwaller J (2017) Aggressive leukemia driven by MLL-AF9. Molecular & Cellular Oncology. https://doi.org/10.1080/23723556.2016.1241854
Bhatlekar S, Fields JZ, Boman BM (2018) Role of HOX genes in stem cell differentiation and cancer. Stem Cells International. https://doi.org/10.1155/2018/3569493
Chen M et al (2019) The fusion oncogene FUS-CHOP drives sarcomagenesis of high-grade spindle cell sarcomas in mice. Sarcoma. https://doi.org/10.1155/2019/1340261
Tornin J et al (2018) FUS-CHOP promotes invasion in myxoid liposarcoma through a SRC/FAK/RHO/ROCK-dependent pathway. Neoplasia 20:44–56. https://doi.org/10.1016/j.neo.2017.11.004
Rodriguez R et al (2013) Expression of FUS-CHOP fusion protein in immortalized/transformed human mesenchymal stem cells drives mixoid liposarcoma formation. Stem Cells. https://doi.org/10.1002/stem.1472
Brunet E et al (2009) Chromosomal translocations induced at specified loci in human stem cells. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.0902076106
Ren YX et al (2008) Mouse mesenchymal stem cells expressing PAX-FKHR form alveolar rhabdomyosarcomas by cooperating with secondary mutations. Can Res. https://doi.org/10.1158/0008-5472.CAN-08-0859
May WA et al (1993) The Ewing’s sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol 13:7393–7398. https://doi.org/10.1128/MCB.13.12.7393
Selvanathan SP et al (2015) Oncogenic fusion protein EWS-FLI1 is a network hub that regulates alternative splicing. Proc Natl Acad Sci 112:E1307–E1316. https://doi.org/10.1073/pnas.1500536112
Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O (2007) Mesenchymal Stem Cell Features of Ewing Tumors. Cancer Cell 11:421–429. https://doi.org/10.1016/j.ccr.2007.02.027
Riggi N et al (2010) EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev 24:916–932. https://doi.org/10.1101/gad.1899710
Soleimani VD, Rudnicki MA (2011) New Insights into the Origin and the Genetic Basis of Rhabdomyosarcomas. Cancer Cell 19:157–159. https://doi.org/10.1016/j.ccr.2011.01.044
Sun X, Guo W, Shen JK, Mankin HJ, Hornicek FJ, Duan Z (2015) Rhabdomyosarcoma: Advances in Molecular and Cellular Biology. Sarcoma. https://doi.org/10.1155/2015/232010
Sorensen PHB et al (2002) PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol 20(11):2672–2679. https://doi.org/10.1200/JCO.2002.03.137
Marshall AD, Grosveld GC (2012) Alveolar rhabdomyosarcoma - The molecular drivers of PAX3/7-FOXO1-induced tumorigenesis. Skeletal Muscle 2(1):1–14. https://doi.org/10.1186/2044-5040-2-25
Xia SJ, Holder DD, Pawel BR, Zhang C, Barr FG (2009) High Expression of the PAX3-FKHR oncoprotein is required to promote tumorigenesis of human myoblasts. Am J Pathol 175(6):2600–2608. https://doi.org/10.2353/ajpath.2009.090192
Murayama T et al (2016) Oncogenic fusion gene CD74-NRG1 confers cancer stem cell-like properties in lung cancer through a IGF2 autocrine/paracrine circuit. Can Res 76(4):974–983. https://doi.org/10.1158/0008-5472.CAN-15-2135
Muscarella LA, Rossi A (2017) NRG1: a cinderella fusion in lung cancer? Lung Cancer Management 6(4):121–123. https://doi.org/10.2217/lmt-2017-0018
Xu C-W et al (2015) Association between EML4-ALK fusion gene and thymidylate synthase mRNA expression in non-small cell lung cancer tissues. Exp Ther Med 9(6):2151–2154. https://doi.org/10.3892/etm.2015.2372
Zhu Y et al (2018) The KIF5B-RET fusion gene mutation as a novel mechanism of acquired EGFR tyrosine kinase inhibitor resistance in lung adenocarcinoma. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2018.09.011
Lei JT et al (2018) Functional annotation of ESR1 gene fusions in estrogen receptor-positive breast cancer. Cell Rep. https://doi.org/10.1016/j.celrep.2018.07.009
Hultsch S (2018) Seven shades of tamoxifen resistance: molecular mechanisms of drug resistance in breast cancer. University of Helsinki. ISBN 978-951-51-4492-8
Veeraraghavan J, Ma J, Hu Y, Wang X-S (2016) Recurrent and pathological gene fusions in breast cancer: current advances in genomic discovery and clinical implications. Breast Cancer Res Treat 158(2):219–232. https://doi.org/10.1007/s10549-016-3876-y
Shih I-M, Kurman RJ (2004) Ovarian Tumorigenesis. Am J Pathol 164(5):1511–1518
Christie EL et al (2019) Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nat Commun. https://doi.org/10.1038/s41467-019-09312-9
Cerrato A, Merolla F, Morra F, Celetti A (2018) CCDC6: the identity of a protein known to be partner in fusion. Int J Cancer 142(7):1300–1308. https://doi.org/10.1002/ijc.31106
Krook MA et al (2019) Tumor heterogeneity and acquired drug resistance in FGFR2-fusion-positive cholangiocarcinoma through rapid research autopsy. Cold Spring Harb Mol Case Stud. https://doi.org/10.1101/mcs.a004002
De Braekeleer E, Douet-Guilbert N, De Braekeleer M (2014) RARA fusion genes in acute promyelocytic leukemia: a review. Expert Rev Hematol 7(3):347–357. https://doi.org/10.1586/17474086.2014.903794
Goto E, Tomita A, Atsumi A, Kiyoi H, Naoe T (2009) Double genetic mutations in PML-rara fusion gene confirmed in a patient showing resistance to all-trans retinoic acid and arsenic-trioxide therapy. Blood 114(22):1743–1743. https://doi.org/10.1182/blood.V114.22.1743.1743
Subramaniyam S et al (2006) Do RARA/PML fusion gene deletions confer resistance to ATRA-based therapy in patients with acute promyelocytic leukemia? Leukemia. https://doi.org/10.1038/sj.leu.2404406
Sun C et al (2008) TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. https://doi.org/10.1038/onc.2008.183
Cerveira N et al (2006) TMPRSS2-ERG Gene Fusion Causing ERG Overexpression Precedes Chromosome Copy Number Changes in Prostate Carcinomas and Paired HGPIN Lesions. Neoplasia 8(10):826–832
Mochmann LH et al (2013) ERG induces a mesenchymal-like state associated with chemoresistance in leukemia cells. Oncotarget 5(2):351–362. https://doi.org/10.18632/oncotarget.1449
Botton T et al (2019) Genetic heterogeneity of BRAF fusion kinases in melanoma affects drug responses. Cell Rep. https://doi.org/10.1016/j.celrep.2019.09.009
Schram AM, Chang MT, Jonsson P, Drilon A (2017) Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 14(12):735–748. https://doi.org/10.1038/nrclinonc.2017.127
Yao T et al (2019) Identification of new fusion genes and their clinical significance in endometrial cancer. Chin Med J 132:1314–1321. https://doi.org/10.1097/CM9.0000000000000203
Willis R (2016) Targeted cancer therapy: Vital oncogenes and a new molecular genetic paradigm for cancer initiation progression and treatment. IJMS 17(9):1552. https://doi.org/10.3390/ijms17091552
Wu C-C, Beird HC, Zhang J, Futreal PA (2018) FusionPathway: Prediction of pathways and therapeutic targets associated with gene fusions in cancer. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1006266
Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD (2007) Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. https://doi.org/10.1038/nrc2126
Muller IB, Langen AJD, Honeywell RJ, Giovannetti E, Peters GJ (2016) Overcoming crizotinib resistance in ALK-rearranged NSCLC with the second-generation ALK-inhibitor ceritinib. Expert Rev Anticancer Ther 16:147–157. https://doi.org/10.1586/14737140.2016.1131612
Ma Y et al (2016) Identification of mutations, gene expression changes and fusion transcripts by whole transcriptome RNAseq in docetaxel resistant prostate cancer cells. Springerplus 5:1861. https://doi.org/10.1186/s40064-016-3543-0
Wang X-S et al (2011) Characterization of KRAS rearrangements in metastatic prostate cancer. Cancer Discov 1:35–43. https://doi.org/10.1158/2159-8274.CD-10-0022
Acknowledgements
The authors would like to thank the SRM Institute of Science and Technology for the infrastructure and fellowship to Mr. Saurav Panicker. The authors express their immense gratitude to Department of Science and Technology (DST), Government of India and the Department of Biotechnology for the funding.
Funding
We would also like to thank the funding support provided to us from Science and Engineering Research Board, a statutory body of Department of Science and Technology (DST), Government of India (EMR/2017/002874), and the Department of Biotechnology, (BT/PR26189/GET/119/226/2017) and SRM Institute of Science and Technology.
Author information
Authors and Affiliations
Contributions
Writing and idea conception, designing and revision: Satish Ramalingam. Writing and designing of the manuscript and interpreting the relevant literature: Saurav Panicker. Provided critical inputs and revising the review appropriately: Sivaramakrishnan Venkatabalasubramanian and Surajit Pathak.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panicker, S., Venkatabalasubramanian, S., Pathak, S. et al. The impact of fusion genes on cancer stem cells and drug resistance. Mol Cell Biochem 476, 3771–3783 (2021). https://doi.org/10.1007/s11010-021-04203-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04203-4