Abstract
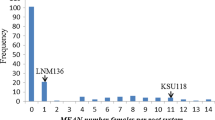
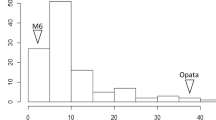
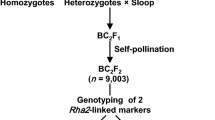
The cereal cyst nematode (CCN, Heterodera avenae Woll.) resistance locus Cre8 on the long arm of chromosome 6B (6BL) of wheat (Triticum aestivum L.) is effective in lowering the nematode population in soil. Identification of reliable and high-throughput molecular markers linked to the Cre8 locus is important for the successful deployment of Cre8-derived resistance in wheat breeding programs. Here, we report the addition of over 600 markers to improve an existing linkage map for the Trident/Molineux wheat population. With the improved map, Cre8 was mapped as a large-effect quantitative trait locus (QTL) near the distal end of 6BL. This QTL explained up to 35 % of the phenotypic variation in CCN resistance. New marker assays were developed for DNA polymorphisms in the Cre8 region. Seven molecular markers closely linked with Cre8 (at 0.9, 2.2 or 5.9 cM from the estimated QTL position) were found to be diagnostic across a panel of wheat cultivars and are recommended for use in marker-assisted selection of the Cre8 resistance locus in wheat breeding. Prospects for the isolation of the causal gene are discussed.



Similar content being viewed by others
References
Akbari M, Wenzl P, Caig V, Carling J, Xia L, Yang S, Uszynski G, Mohler V, Lehmensiek A, Kuchel H, Hayden M, Howes N, Sharp P, Vaughan P, Rathmell B, Huttner E, Kilian A (2006) Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet 113(8):1409–1420. doi:10.1007/s00122-006-0365-4
Akhunov ED, Akhunova AR, Anderson OD, Anderson JA, Blake N, Clegg MT, Coleman-Derr D, Conley EJ, Crossman CC, Deal KR, Dubcovsky J, Gill BS, Gu YQ, Hadam J, Heo H, Huo N, Lazo GR, Luo M-C, Ma YQ, Matthews DE, McGuire PE, Morrell PL, Qualset CO, Renfro J, Tabanao D, Talbert LE, Tian C, Toleno DM, Warburton ML, You FM, Zhang W, Dvorak J (2010) Nucleotide diversity maps reveal variation in diversity among wheat genomes and chromosomes. BMC Genom 11(1):702. doi:10.1186/1471-2164-11-702
Allen AM, Barker GLA, Wilkinson P, Burridge A, Winfield M, Coghill J, Uauy C, Griffiths S, Jack P, Berry S, Werner P, Melichar JPE, McDougall J, Gwilliam R, Robinson P, Edwards KJ (2012) Discovery and development of exome-based, co-dominant single nucleotide polymorphism markers in hexaploid wheat (Triticum aestivum L.). Plant Biotechnol J 11(3):279–295. doi:10.1111/pbi.12009
Banyer RJ, Fisher JM (1971) Seasonal variation in hatching of eggs of Heterodera Avenae. Nematologica 17(2):225–236. doi:10.1163/187529271X00071
Cai D, Kleine M, Kifle S, Harloff H-J, Sandal NN, Marcker KA, Klein-Lankhorst RM, Salentijn EMJ, Lange W, Stiekema WJ, Wyss U, Grundler FMW, Jung C (1997) Positional cloning of a gene for nematode resistance in sugar beet. Science 275(5301):832–834. doi:10.1126/science.275.5301.832
Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, See D, Bai G, Pumphrey M, Tomar L, Wong D, Kong S, Reynolds M, da Silva ML, Bockelman H, Talbert L, Anderson JA, Dreisigacker S, Baenziger S, Carter A, Korzun V, Morrell PL, Dubcovsky J, Morell MK, Sorrells ME, Hayden MJ, Akhunov E (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci USA 110(20):8057–8062. doi:10.1073/pnas.1217133110
Crossa J, Burgueño J, Dreisigacker S, Vargas M, Herrera-Foessel SA, Lillemo M, Singh RP, Trethowan R, Warburton M, Franco J, Reynolds M, Crouch JH, Ortiz R (2007) Association analysis of historical bread wheat germplasm using additive genetic covariance of relatives and population structure. Genetics 177(3):1889–1913. doi:10.1534/genetics.107.078659
de Majnik J, Ogbonnaya FC, Moullet O, Lagudah ES (2003) The Cre1 and Cre3 nematode resistance genes are located at homeologous loci in the wheat genome. Mol Plant Microbe Interact 16(12):1129–1134. doi:10.1094/MPMI.2003.16.12.1129
Delibes A, Romero D, Aguaded S, Duce A, Mena M, Lopez-Braña I, Andrés MF, Martin-Sanchez JA, García-Olmedo F (1993) Resistance to the cereal cyst nematode (Heterodera avenae Woll.) transferred from the wild grass Aegilops ventricosa to hexaploid wheat by a “stepping-stone” procedure. Theor Appl Genet 87(3):402–408. doi:10.1007/bf01184930
Eastwood R, Lagudah E, Appels R, Hannah M, Kollmorgen J (1991) Triticum tauschii: a novel source of resistance to cereal cyst nematode (Heterodera avenae). Aust J Agric Res 42(1):69–77. doi:10.1071/AR9910069
Eastwood RF, Lagudah ES, Appels R (1994) A directed search for DNA sequences tightly linked to cereal cyst nematode resistance genes in Triticum tauschii. Genome 37(2):311–319. doi:10.1139/g94-043
Ernst K, Kumar A, Kriseleit D, Kloos D-U, Phillips MS, Ganal MW (2002) The broad-spectrum potato cyst nematode resistance gene (Hero) from tomato is the only member of a large gene family of NBS–LRR genes with an unusual amino acid repeat in the LRR region. Plant J 31(2):127–136. doi:10.1046/j.1365-313X.2002.01341.x
Fisher JM (1982) Towards a consistent laboratory assay for resistance to Heterodera avenae. Bull OEPP 12(4):445–449. doi:10.1111/j.1365-2338.1982.tb01828.x
Hayden M, Nguyen T, Waterman A, Chalmers K (2008) Multiplex-ready PCR: a new method for multiplexed SSR and SNP genotyping. BMC Genom 9(1):80. doi:10.1186/1471-2164-9-80
Jahier J, Tanguy AM, Abelard P, Rivoal R (1996) Utilization of deletions to localize a gene for resistance to the cereal cyst nematode, Heterodera avenue, on an Aegilops ventricosa chromosome. Plant Breed 115(4):282–284. doi:10.1111/j.1439-0523.1996.tb00919.x
Jahier J, Abelard P, Tanguy M, Dedryver F, Rivoal R, Khatkar S, Bariana HS, Koebner R (2001) The Aegilops ventricosa segment on chromosome 2AS of the wheat cultivar ‘VPM1’ carries the cereal cyst nematode resistance gene Cre5. Plant Breed 120(2):125–128. doi:10.1046/j.1439-0523.2001.00585.x
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12(1):172–175. doi:10.1111/j.1469-1809.1943.tb02321.x
Kuchel H, Langridge P, Mosionek L, Williams K, Jefferies SP (2006) The genetic control of milling yield, dough rheology and baking quality of wheat. Theor Appl Genet 112(8):1487–1495. doi:10.1007/s00122-006-0252-z
Lagudah ES, Moullet O, Appels R (1997) Map-based cloning of a gene sequence encoding a nucleotide-binding domain and a leucine-rich region at the Cre3 nematode resistance locus of wheat. Genome 40(5):659–665. doi:10.1139/g97-087
Lewis JG, Matic M, McKay AC (2009) Success of cereal cyst nematode resistance in Australia: history and status of resistance screening systems. In: Riley IT, Nicol JM, Dababat AA (eds) Cereal cyst nematodes: status, research and outlook. Proceedings of the first workshop of the international cereal cyst nematode initiative CIMMYT, Ankara, Turkey, pp 137–142
Manly KF, Cudmore RH Jr, Meer JM (2001) Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome 12(12):930–932. doi:10.1007/s00335-001-1016-3
Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10(8):1307. doi:10.1105/tpc.10.8.1307
Murray GM, Brennan JP (2009) The current and potential costs from diseases of wheat in Australia. Grains Research and Development Corporation, Australia
Ogbonnaya FC, Seah S, Delibes A, Jahier J, López-Braña I, Eastwood RF, Lagudah ES (2001) Molecular-genetic characterisation of a new nematode resistance gene in wheat. Theor Appl Genet 102(4):623–629. doi:10.1007/s001220051689
Ophel-Keller K, McKay A, Hartley D, Curran J (2008) Development of a routine DNA-based testing service for soilborne diseases in Australia. Australas Plant Pathol 37(3):243–253. doi:10.1071/AP08029
Paal J, Henselewski H, Muth J, Meksem K, Menéndez CM, Salamini F, Ballvora A, Gebhardt C (2004) Molecular cloning of the potato Gro1–4 gene conferring resistance to pathotype Ro1 of the root cyst nematode Globodera rostochiensis, based on a candidate gene approach. Plant J 38(2):285–297. doi:10.1111/j.1365-313X.2004.02047.x
Pallotta MA, Graham RD, Langridge P, Sparrow DHB, Barker SJ (2000) RFLP mapping of manganese efficiency in barley. Theor Appl Genet 101(7):1100–1108. doi:10.1007/s001220051585
Paull JG, Chalmers KJ, Karakousis A, Kretschmer JM, Manning S, Langridge P (1998) Genetic diversity in Australian wheat varieties and breeding material based on RFLP data. Theor Appl Genet 96(3):435–446. doi:10.1007/s001220050760
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ranjbar GA (1997) Production and utilization of doubled haploid lines in wheat breeding programs. PhD Thesis, The University of Adelaide, Australia
Rogowsky P, Guidet F, Langridge P, Shepherd K, Koebner R (1991) Isolation and characterization of wheat–rye recombinants involving chromosome arm 1DS of wheat. Theor Appl Genet 82(5):537–544. doi:10.1007/BF00226788
Romero MD, Montes MJ, Sin E, Lopez-Braña I, Duce A, Martín-Sanchez JA, Andrés MF, Delibes A (1998) A cereal cyst nematode (Heterodera avenae Woll.) resistance gene transferred from Aegilops triuncialis to hexaploid wheat. Theor Appl Genet 96(8):1135–1140. doi:10.1007/s001220050849
Rozen S, Skaletsky H (1999) Primer3 on the WWW for general users and for biologist programmers. In: Misener S, Krawetz S (eds) Bioinformatics methods and protocols, vol 132., Methods in molecular biology™Humana Press, New York, pp 365–386 10.1385/1-59259-192-2:365
Sears ER (1954) The aneuploids of common wheat. Res Bull Mo Agric Exp Sta 572:1–58
Slootmaker LAJ, Lange W, Jochemsen G, Schepers J (1974) Monosomic analysis in bread wheat of resistance to cereal root eelworm. Euphytica 23(3):497–503. doi:10.1007/bf00022470
Somers D, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109(6):1105–1114. doi:10.1007/s00122-004-1740-7
Tanaka T, Kobayashi F, Joshi GP, Onuki R, Sakai H, Kanamori H, Wu J, Šimková H, Nasuda S, Endo TR, Hayakawa K, Doležel J, Ogihara Y, Itoh T, Matsumoto T, Handa H (2013) Next-generation survey sequencing and the molecular organization of wheat chromosome 6B. DNA Res. doi:10.1093/dnares/dst041
Trick M, Adamski NM, Mugford SG, Jiang CC, Febrer M, Uauy C (2012) Combining SNP discovery from next-generation sequencing data with bulked segregant analysis (BSA) to fine-map genes in polyploid wheat. BMC Plant Biol 12:14. doi:10.1186/1471-2229-12-14
Van Os H, Stam P, Visser RGF, Van Eck HJ (2005) RECORD: a novel method for ordering loci on a genetic linkage map. Theor Appl Genet 112(1):30–40. doi:10.1007/s00122-005-0097-x
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93(1):77–78. doi:10.1093/jhered/93.1.77
Wang S, Basten C, Zeng Z (2012) Windows QTL cartographer 2.5. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Williams KJ, Fisher JM, Langridge P (1994) Identification of RFLP markers linked to the cereal cyst nematode resistance gene (Cre) in wheat. Theor Appl Genet 89(7):927–930. doi:10.1007/bf00224519
Williams KJ, Lewis JG, Bogacki P, Pallotta MA, Willsmore KL, Kuchel H, Wallwork H (2003) Mapping of a QTL contributing to cereal cyst nematode tolerance and resistance in wheat. Aust J Agric Res 54(8):731–737. doi:10.1071/AR02225
Williams K, Willsmore K, Olson S, Matic M, Kuchel H (2006) Mapping of a novel QTL for resistance to cereal cyst nematode in wheat. Theor Appl Genet 112(8):1480–1486. doi:10.1007/s00122-006-0251-0
Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ (2003) High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem 49(6):853–860. doi:10.1373/49.6.853
You F, Huo N, Gu Y, Luo M-C, Ma Y, Hane D, Lazo G, Dvorak J, Anderson O (2008) BatchPrimer3: a high throughput web application for PCR and sequencing primer design. BMC Bioinformatics 9(1):253. doi:10.1186/1471-2105-9-253
Acknowledgments
This work was supported by grants from the Grains Research and Development Corporation and by a University of Adelaide International Postgraduate Research Scholarship awarded to Dimanthi Jayatilake. We thank Peter Langridge for providing the plasmid DNA for the AWBMA20 clone and Beata Sznajder for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dimanthi V. Jayatilake and Elise J. Tucker are joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jayatilake, D.V., Tucker, E.J., Brueggemann, J. et al. Genetic mapping of the Cre8 locus for resistance against cereal cyst nematode (Heterodera avenae Woll.) in wheat. Mol Breeding 35, 66 (2015). https://doi.org/10.1007/s11032-015-0235-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0235-3