Abstract
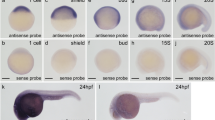
Atypical Rett syndrome is a child neurodevelopmental disorder induced by mutations in CDKL5 gene and characterized by a progressive regression in development with loss of purposeful use of the hands, slowed brain and head growth, problems with walking, seizures, and intellectual disability. At the moment, there is no cure for this pathology and little information is available concerning animal models capable of mimicking its phenotypes, thus the development of additional animal models should be of interest to gain more knowledge about the disease. Zebrafish has been used successfully as model organism for many human genetic diseases; however, no information is available concerning the spatial and temporal expression of cdkl5 orthologous in this organism. In the present study, we identified the developmental expression patterns of cdkl5 in zebrafish by quantitative PCR and whole-mount in situ hybridization. cdkl5 is expressed maternally at low levels during the first 24 h of development. After that the expression of the gene increases significantly and it starts to be expressed mainly in the nervous system and in several brain structures, such as telencephalon, mesencephalon and diencephalon. The expression patterns of cdkl5 in zebrafish is in accordance with the tissues known to be affected in humans and associated to symptoms and deficits observed in Rett syndrome patients thus providing the first evidence that zebrafish could be an alternative model to study the molecular pathways of this disease as well as to test possible therapeutic approaches capable of rescuing the phenotype.



Similar content being viewed by others
References
Moretti P, Zoghbi HY (2006) MeCP2 dysfunction in Rett syndrome and related disorders. Curr Opin Genet Dev 16(3):276–281. https://doi.org/10.1016/j.gde.2006.04.009
Wang ITJ, Allen M, Goffin D, Zhu X, Fairless AH, Brodkin ES, Siegel SJ, Marsh ED, Blendy JA, Zhou Z (2012) Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc Natl Acad Sci USA 109(52):21516–21521. https://doi.org/10.1073/pnas.1216988110
Amendola E, Zhan Y, Mattucci C, Castroflorio E, Calcagno E, Fuchs C, Lonetti G, Silingardi D, Vyssotski AL, Farley D, Ciani E, Pizzorusso T, Giustetto M, Gross CT (2014) Mapping pathological phenotypes in a mouse model of CDKL5 disorder. PLoS ONE 9(5):e91613. https://doi.org/10.1371/journal.pone.0091613
Okuda K, Kobayashi S, Fukaya M, Watanabe A, Murakami T, Hagiwara M, Sato T, Ueno H, Ogonuki N, Komano-Inoue S, Manabe H, Yamaguchi M, Ogura A, Asahara H, Sakagami H, Mizuguchi M, Manabe T, Tanaka T (2017) CDKL5 controls postsynaptic localization of GluN2B-containing NMDA receptors in the hippocampus and regulates seizure susceptibility. Neurobiol Dis 106(Supplement C):158–170. https://doi.org/10.1016/j.nbd.2017.07.002
Dooley K, Zon LI (2000) Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 10(3):252–256. https://doi.org/10.1016/S0959-437X(00)00074-5
Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8(5):353–367. https://doi.org/10.1038/nrg2091
Khan KM, Collier AD, Meshalkina DA, Kysil EV, Khatsko SL, Kolesnikova T, Morzherin YY, Warnick JE, Kalueff AV, Echevarria DJ (2017) Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br J Pharmacol 174(13):1925–1944. https://doi.org/10.1111/bph.13754
Amar A (2017) Commentary: zebrafish as a model for epilepsy-induced cognitive dysfunction: a pharmacological, biochemical and behavioral approach. Front Pharmacol 8:851. https://doi.org/10.3389/fphar.2017.00851
Yun-Zhu P, Liang L, Ai-Ling F, Yan L, Lan S, Qian L, Dan W, Man-Ji S, Ying-Ge Z, Bao-Quan Z (2017) Generation of alzheimer’s disease transgenic zebrafish expressing human APP mutation under control of zebrafish appb promotor. Curr Alzheimer Res 14(6):668–679. https://doi.org/10.2174/1567205013666161201202000
Matsui H, Takahashi R (2017) Parkinson’s disease pathogenesis from the viewpoint of small fish models. J Neural Transm. https://doi.org/10.1007/s00702-017-1772-1
Giacomotto J, Carroll AP, Rinkwitz S, Mowry B, Cairns MJ, Becker TS (2016) Developmental suppression of schizophrenia-associated miR-137 alters sensorimotor function in zebrafish. Transl Psychiatry 6(5):e818. https://doi.org/10.1038/tp.2016.88
Marcon M, Herrmann AP, Mocelin R, Rambo CL, Koakoski G, Abreu MS, Conterato GMM, Kist LW, Bogo MR, Zanatta L, Barcellos LJG, Piato AL (2016) Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology 233(21):3815–3824. https://doi.org/10.1007/s00213-016-4408-5
Gerlai R (2015) Embryonic alcohol exposure: towards the development of a zebrafish model of fetal alcohol spectrum disorders. Dev Psychobiol 57(7):787–798. https://doi.org/10.1002/dev.21318
Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241(4861):42–52. https://doi.org/10.1126/science.3291115
Manning G, Plowman GD, Hunter T, Sudarsanam S (2002) Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci 27(10):514–520. https://doi.org/10.1016/s0968-0004(02)02179-5
Chatonnet F, Thoby-Brisson M, Abadie V, Domínguez del Toro E, Champagnat J, Fortin G (2002) Early development of respiratory rhythm generation in mouse and chick. Respir Physiol Neurobiol 131(1–2):5–13. https://doi.org/10.1016/S1569-9048(02)00033-2
Nozaki S, Iriki A, Nakamura Y (1986) Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol 55(4):806–825. https://doi.org/10.1152/jn.1986.55.4.806
Breedlove SM, Watson NV, Rosenzweig MR (2010) Biological psychology: an introduction to behavioral, cognitive, and clinical neuroscience. 6th edn, Sinauer Associates, Inc., Sunderland
Wray S, Fueshko SM, Kusano K, Gainer H (1996) GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol 180(2):631–645. https://doi.org/10.1006/dbio.1996.0334
Hemsley KM, Crocker AD (2001) Changes in muscle tone are regulated by D1 and D2 dopamine receptors in the Ventral striatum and D1 receptors in the substantia Nigra. Neuropsychopharmacology 25(4):514–526. https://doi.org/10.1016/S0893-133X(01)00245-7
Arnould-taylor WE (1998) A textbook of anatomy and physiology. 3rd edn, Stanley Thornes, Ltd., Cheltenham
Hector RD, Dando O, Landsberger N, Kilstrup-Nielsen C, Kind PC, Bailey MES, Cobb SR (2016) Characterisation of CDKL5 transcript isoforms in human and mouse. PLoS ONE 11(6):e0157758. https://doi.org/10.1371/journal.pone.0157758
Bahi-Buisson N, Nectoux J, Rosas-Vargas H, Milh M, Boddaert N, Girard B, Cances C, Ville D, Afenjar A, Rio M, Héron D, N’Guyen Morel MA, Arzimanoglou A, Philippe C, Jonveaux P, Chelly J, Bienvenu T (2008) Key clinical features to identify girls with CDKL5 mutations. Brain 131(10):2647–2661. https://doi.org/10.1093/brain/awn197
Krishnaraj R, Ho G, Christodoulou J (2017) RettBASE: Rett syndrome database update. Hum Mutat 38(8):922–931. https://doi.org/10.1002/humu.23263
Evans JC, Archer HL, Colley JP, Ravn K, Nielsen JB, Kerr A, Williams E, Christodoulou J, Gécz J, Jardine PE, Wright MJ, Pilz DT, Lazarou L, Cooper DN, Sampson JR, Butler R, Whatley SD, Clarke AJ (2005) Early onset seizures and Rett-like features associated with mutations in CDKL5. Eur J Hum Genet 13:1113. https://doi.org/10.1038/sj.ejhg.5201451. https://www.nature.com/articles/5201451#supplementary-information
Fehr S, Downs J, Ho G, de Klerk N, Forbes D, Christodoulou J, Williams S, Leonard H (2016) Functional abilities in children and adults with the CDKL5 disorder. Am J Med Genet A 170(11):2860–2869. https://doi.org/10.1002/ajmg.a.37851
Archer HL, Evans J, Edwards S, Colley J, Newbury-Ecob R, O’Callaghan F, Huyton M, O’Regan M, Tolmie J, Sampson J, Clarke A, Osborne J (2006) CDKL5 mutations cause infantile spasms, early onset seizures, and severe mental retardation in female patients. J Med Genet 43(9):729–734. https://doi.org/10.1136/jmg.2006.041467
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203(3):253–310. https://doi.org/10.1002/aja.1002030302
Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3(1):59–69. https://doi.org/10.1038/nprot.2007.514
Acknowledgements
This research was partially supported by national funds from FCT - Foundation for Science and Technology through project UID/Multi/04326/2013. MV was supported by a postdoctoral fellowship reference SFRH/BPD/65923/2009 from the Portuguese Foundation for Science and Technology (FCT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures involving animals followed the European Directive 2010/63/EU and the related guidelines (European Commission, 2014) and Portuguese legislation (Decreto-Lei 113/2013 and Despacho 2880/2015) for animal experimentation and welfare.
Rights and permissions
About this article
Cite this article
Vitorino, M., Cunha, N., Conceição, N. et al. Expression pattern of cdkl5 during zebrafish early development: implications for use as model for atypical Rett syndrome. Mol Biol Rep 45, 445–451 (2018). https://doi.org/10.1007/s11033-018-4180-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4180-1