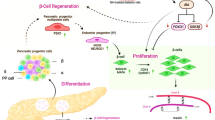
Abstract
In the quest to understand lost β-cells regeneration in the diabetic condition, we have demonstrated successful differentiation of human haematopoietic stem cells (HSCs) to functional β-like cells. Costus igneus (Ci) leaf extract is known to exhibit anti-diabetic properties by lowering the blood glucose level as demonstrated in mice models. To establish the anti-diabetic properties of Ci leaf extract on human subjects, we studied the effect of Ci on these differentiated β-like cells. Ci leaf extract showed its anti-diabetic property through elevated glucokinase activity which catalyzes the rate-limiting step of glucose catabolism in β-like cells and acts as a sensor for insulin production while decreasing the glucose-6-phosphatase activity. Upon increasing the concentrations of Ci leaf extract (25, 65, 105, 145, 185 µg/ml) and glucose concentrations (5.5, 11.1, and 25 mM) Ci leaf extract treated β-like cells showed enhanced glucokinase and decreased glucose-6-phosphatase activities and an exponential rise in gene expressions of INS and GLUT2 was observed. The present study shows enhanced INS and GLUT2 gene expression and elevated glucokinase activity in β-like cells differentiated from HSCs upon treatment with Ci leaf extract explain the anti-diabetic property of Ci leaf extract. This extract can be effectively used in the management of diabetes.





Similar content being viewed by others
Data availability
Yes.
References
Lee J, Yoon SR, Choi I, Jung H (2019) Causes and mechanisms of hematopoietic stem cell aging. Int J Mol Sci. https://doi.org/10.3390/ijms20061272
Zimmermann S, Martens UM (2008) Telomeres, senescence, and hematopoietic stem cells. Cell Tissue Res. https://doi.org/10.1007/s00441-007-0469-4
Kanji S, Pompili VJ (2012) Plasticity and maintenance of hematopoietic stem cells during development. Recent aPat Biotechnol. https://doi.org/10.2174/187220811795655896
Sunitha MM, Srikanth L, Kumar PS, Chandrasekhar C, Sarma PVGK (2016) Role of STPK in maintaining the quiescent nature of human CD34+ stem cells. J Stem Cells 11(3):125
Sarma PVGK, Subramanyam G (2008) In vitro cardiogenesis can be initiated in human CD34+ cells. Indian Heart J 60(2):95–100
Takahashi A, Nagashima K, Hamasaki A, Kuwamura N, Kawasaki Y, Ikeda H, Yamada Y, Inagaki N, Seino Y (2007) Sulfonylurea and glinide reduce insulin content, functional expression of KATP channels, and accelerate apoptotic β-cell death in the chronic phase. Diabetes Res Clin Pract 77(3):343–350. https://doi.org/10.1016/j.diabres.2006.12.021
Kozlovski P, Bhosekar V, Foley JE (2017) DPP-4 inhibitor treatment: β-cell response but not HbA1c reduction is dependent on the duration of diabetes. Vasc Health Risk Manag 13:123–126. https://doi.org/10.2147/VHRM.S125850
Sunitha MM, Srikanth L, Santhosh Kumar P, Chandrasekhar C, Sarma PVGK (2016) In vitro differentiation potential of human haematopoietic CD34+ cells towards pancreatic β-cells. Cell Biol Int 40(10):1084–1093. https://doi.org/10.1002/cbin.10654
Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A (2001) β-cell adaptation and decompensation during the progression of diabetes. Diabetes. https://doi.org/10.2337/diabetes.50.2007.s154
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev. https://doi.org/10.1152/physrev.00063.2017
Alves C, Casqueiro J, Casqueiro J (2012) Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. https://doi.org/10.4103/2230-8210.94253
Rizvi SI, Mishra N (2013) Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res. https://doi.org/10.1155/2013/712092
Joshi BN, Munot H, Hardikar M, Kulkarni AA (2013) Orally active hypoglycemic protein from Costus igneus N. E. Br.: an in vitro and in vivo study. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2013.05.093
Shetty AJ, Choudhury D, Rejeesh VN, Kuruvilla M, Kotian S (2010) Effect of the insulin plant (Costus igneus) leaves on dexamethasone-induced hyperglycemia. Int J Ayurveda Res 1(2):100–102. https://doi.org/10.4103/0974-7788.64396
Thorens B (2015) GLUT2, glucose sensing and glucose homeostasis. Diabetologia 58(2):221–232. https://doi.org/10.1007/s00125-014-3451-1
Agius L (2009) Targeting hepatic glucokinase in type 2 diabetes: weighing the benefits and risks. Diabetes 58(1):18–20. https://doi.org/10.2337/db08-1470
Srikanth L, Sunitha MM, Venkatesh K, Kumar PS, Chandrasekhar C, Vengamma B, Sarma PVGK (2015) Anaerobic glycolysis and HIFα expression in haematopoietic stem cells explains its quiescence nature. J Stem Cells 10(2):97
Annegowda HV, Anwar LN, Mordi MN, Ramanathan S, Mansor SM (2010) Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacogn Res. https://doi.org/10.4103/0974-8490.75457
Yellapu NK, Valasani KR, Pasupuleti SK, Gopal S, Sarma PVGK, Matcha B (2014) Identification and analysis of novel R308K mutation in glucokinase of type 2 diabetic patient and its kinetic correlation. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1209
Plummer DT (2008) An introduction to practical biochemistry. TATA McGraw-Hill Education, New York
Venkatesh K, Srikanth L, Vengamma B, Chandrasekhar C, Sanjeevkumar A, Mouleshwara Prasad BC, Sarma PVGK (2013) In vitro differentiation of cultured human CD34+ cells into astrocytes. Neurol India. https://doi.org/10.4103/0028-3886.117615
Gloyn AL (2003) Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat. https://doi.org/10.1002/humu.10277
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Kaabi JA (2020) Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. https://doi.org/10.2991/JEGH.K.191028.001
Salehi B, Ata A, Kumar NVA, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Ayatollahi SA, Fokou PVT, Kobarfard F, Zakaria ZA, Iriti M, Taheri Y, Martorell M, Sureda A, Setzer WN, Durazzo A, Lucarini M, Santini A, Capasso R, Sharifi-Rad J (2019) Antidiabetic potential of medicinal plants and their active components. Biomolecules. https://doi.org/10.3390/biom9100551
Sikirica MV, Martin AA, Wood R, Leith A, Piercy J, Higgins V (2017) Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes 10:403–412. https://doi.org/10.2147/DMSO.S141235
Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M (2016) The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician. https://doi.org/10.19082/1832
Hegde P, Rao H, Rao P (2014) A review on insulin plant (Costus igneus Nak). Pharmacogn Rev. https://doi.org/10.4103/0973-7847.125536
Darmawan D (2019) Costus igneus—a therapeutic anti-diabetic herb with active phytoconstituents. J Chem Inf Model. https://doi.org/10.13040/IJPSR.0975-8232.10(8).3583-91
Miura T, Itoh Y, Kaneko T, Ueda N, Ishida T, Fukushima M, Matsuyama F, Seino Y (2004) Corosolic acid induces GLUT4 translocation in genetically type 2 diabetic mice. Biol Pharm Bull. https://doi.org/10.1248/bpb.27.1103
Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol. https://doi.org/10.3389/fendo.2013.00037
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R (2015) Type 2 diabetes mellitus. Nat Rev Dis Primers. https://doi.org/10.1038/nrdp.2015.19
Röder PV, Wu B, Liu Y, Han W (2016) Pancreatic regulation of glucose homeostasis. Exp Mol Med. https://doi.org/10.1038/emm.2016.6
Gireesh G, Thomas SK, Joseph B, Paulose CS (2009) Antihyperglycemic and insulin secretory activity of Costus pictus leaf extract in streptozotocin induced diabetic rats and in in vitro pancreatic islet culture. J Ethnopharmacol 123:470–474
Funding
We acknowledge Sri Venkateswara Institute of Medical Sciences for providing facilities to carry out the work.
Author information
Authors and Affiliations
Contributions
Work design editing of manuscript: PVGKS. Treating of Costus igneus to in vitro differentiated β-like cells, Writing of Manuscript: SK. Characterization of in vitro differentiated β-like cells: SMM. Characterization of Human HSCs: SEL, SK.
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there is no conflict of interest upon publication of this paper.
Ethical approval
The Institutional Ethics committee approved the research protocol (IEC No: 31/06/2006 and 419/27–01-2015).
Consent to participate
Blood donor has given written informed consent for his participation in the study.
Consent for publication
All authors concurred with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kattaru, S., Manne Mudhu, S., Echambadi Loganathan, S. et al. Increased insulin and GLUT2 gene expression and elevated glucokinase activity in β-like cells of islets of langerhans differentiated from human haematopoietic stem cells on treatment with Costus igneus leaf extract. Mol Biol Rep 48, 4477–4485 (2021). https://doi.org/10.1007/s11033-021-06467-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06467-x