Abstract
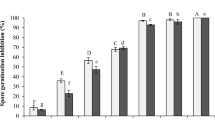
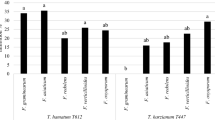
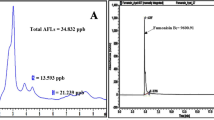
This study was undertaken to evaluate the effect of Ephedra major Host, an important medicinal plant with various biological activities, on growth and aflatoxin (AF) production by Aspergillus parasiticus NRRL 2999. The fungus was cultured in yeast extract-sucrose (YES) broth, a conductive medium that supports AF production, in the presence of various concentrations of essential oil (EO), hexanic and methanolic extracts of plant aerial parts, fruits, and roots using microbioassay technique. After incubating for 96 h at 28°C in static conditions, mycelial dry weight was determined as an index of fungal growth, and aflatoxin B1 (AFB1) was measured using HPLC technique. Based on the obtained results, EO of plant aerial parts significantly inhibited fungal growth at the highest concentration of 1000 μg/ml without any obvious effect on AFB1 production at all concentrations used. Among plant extracts tested, only methanolic extract of aerial parts and roots were found to inhibit fungal growth and AFB1 production dose-dependently with an IC50 value of 559.74 and 3.98 μg/ml for AFB1, respectively. Based on the GC/MS data, the major components of E. major EO were bis (2-ethylhexyl) phthalate (42.48%), pentacosane (20.94%), docosane (14.64%), citronellol (5.15%), heptadecan (4.41%), cis-3-Hexen-1-ol benzoate (4.07%), and 7-Octen-2-ol (3.25%). With respect to the potent inhibition of fungal growth and AF production by E. major, this plant may be useful in protecting crops from both toxigenic fungal growth and AF contamination.
Similar content being viewed by others
References
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153:1677–92.
Kirk GD, Bah E, Montesano R. Molecular epidemiology of human liver cancer: insights into etiology, pathogenesis and prevention from The Gambia, West Africa. Carcinogenesis. 2006;27:2070–82.
Zaika LL, Buchanan RL. Review of compounds affecting the biosynthesis or bio-regulation of aflatoxins. J Food Prot. 1987;50:691–708.
Sakuda S, Ono M, Ikeda H, Nakamura T, Inagaki Y, Kawachi R, et al. Blasticidin A as an inhibitor of aflatoxin production by Aspergillus parasiticus. J Antibiot (Tokyo). 2000;68:407–12.
Rasooli I, Razzaghi-Abyaneh M. Inhibitory effects of Thyme oils on growth and aflatoxin production by Aspergillus parasiticus. Food Control. 2004;15:479–83.
Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Eslamifar A, Schmidt OJ, Gharebaghi R, Karimian M, et al. Inhibitory effects of Akacid®plus on growth and aflatoxin production by Aspergillus parasiticus. Mycopathologia. 2006;161:245–9.
Razzaghi-Abyaneh M, Yoshinari T, Shams-Ghahfarokhi M, Rezaee MB, Nagasawa H, Sakuda S. Dillapiol and apiol as specific inhibitors for the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci Biotechnol Biochem. 2007;71:2329–32.
Yoshinari T, Akiyama T, Nakamura K, Kondo T, Takahashi Y, Muraoka Y, et al. Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology. 2007;153:2774–80.
Samy RP, Gopalakrishnakone P. Therapeutic potential of plants as anti-microbials for drug discovery. Evid-Based Compl Alter Med. 2008;eCAM:1–12.
Samuelsson G. Drugs of natural origin: a textbook of pharmacognosy. Stockholm: 5th Swedish Pharmaceutical Press; 2004.
Bidlack WR, Omaye ST, Meskin MS, Topham D. Phytochemicals as bioactive agents. Lancaster, UK: Technomic Publishing Company; 2000. p. 106–110.
Stevenson DW. Ephedraceae. In: Flora of North America Editorial Committee, editor. Flora of North America, vol. 2. New York: Oxford University Press; 1993. p. 428–34.
Price RA. Systematics of the gnetales: a review of morphological and molecular evidence. Int J Plant Sci. 1996;157:S40–9.
Anonymous. The Ephedras. Lawrence Review of Natural Products. MO: St. Louis; 1995. p. 1–2.
Soni MG, Carabin IJ, Griffiths JC, Burdock GA. Safety of Ephedra: lessons learned. Toxicol Lett. 2004;150:97–110.
Miyazawa M, Minamino Y, Kameoka H. Volatile components of Ephedra sinica stapf. Flav Fragr J. 1997;12:15–7.
Wang Q, Yang Y, Zhao X. Chemical variation in the essential oil of Ephedra sinica from Northeastern China. Food Chem. 2006;98:52–8.
Tricker AR, Wacker CD, Preussmann R. 2-(N-nitroso-N-methylamino) propiophenone, a direct acting bacterial mutagen found in nitrosated Ephedra altissima tea. Toxicol Lett. 1987;38:45–50.
Al-Khalil S. Transtorine, a new quinoline alkaloid from Ephedra transitoria. J Natl Prod. 1998;61:262–3.
Cottiglia F, Bonsignore L, Casu L, Deidda D. Phenolic constituents from Ephedra nebrodensis. Natl Prod Res. 2005;19:117–23.
Feresin GE, Tapia A, Lopez SN, Zacchino SA. Antimicrobial activity of plants used in traditional medicine of Sun Juan Province, Argentine. J Ethnopharmacol. 2001;78:103–7.
Gurley BJ, Wang P, Gardner SF. Ephedrine-type alkaloid content of nutritional supplements containing Ephedra sinica (ma-huang) as determined by high performance liquid chromatography. J Pharmacol Sci. 1998;87:1547–53.
Ghahreman A. Basic Botany. Tehran: Tehran University Publication; 1994. p. 443–50.
Davies NW. Gas chromatographic retention index of monoterpenes and sesquiterpenes on methyl silicone and carbowax 20 M phases. J Chromatogr. 1998;503:1–24.
Ozdemir G, Karabay NU, Dalay MC, Pazarbasi B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother Res. 2004;18:754–8.
Rosato A, Vitali C, De Laurentis N. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine. 2007;14:727–32.
Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rezaee MB, Jaimand K, Alinezhad S, Saberi R, et al. Chemical composition, antiaflatoxigenic activity of Carum carvi L. Thymus vulgaris and Citrus aurantifolia essential oils. Food Control. 2009;20:1018–24.
Rameshthangam P, Ramasamy P. Antiviral activity bis (2-methylheptyl) phthalate isolated from Pongamia pinnata leaves against white spot syndrome virus of Penaeus monodon Fabricus. Virus Res. 2007;126:38–44.
Fazly Bazzaz BS, Haririzadeh G. Screening of Iranian plants for antimicrobial activity. Pharm Biol. 2003;41:573–83.
Aziz NH, Farag SE, Mousa LA, Abo-Zaid MA. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93:43–54.
Kim JH, Campbell BC, Mahoney NE, Chan KL, Molyneux RJ. Identification of phenolics for control of Aspergillus flavus using Saccharomyces cerevisiae in a model target-gene bioassay. J Agric Food Chem. 2004;52:7814–21.
Nawwar MAM, El-Sissi HI, Barakat HH. Flavonoid constituents of Ephedra alata. Phytochemistry. 1984;23:2937–9.
Nawwar MAM, Barakat HH, Buddrust J, Linscheidt M. Alkaloidal, lignan and phenolic constituents of Ephedra alata. Phytochemistry. 1985;24:878–9.
Acknowledgments
The present work was financially supported by Pasteur Institute of Iran. The authors wish to thank Afsaneh Dehnamaki from Mycology Department of Pasteur Institute of Iran for her helpful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagheri-Gavkosh, S., Bigdeli, M., Shams-Ghahfarokhi, M. et al. Inhibitory Effects of Ephedra major Host on Aspergillus parasiticus Growth and Aflatoxin Production. Mycopathologia 168, 249–255 (2009). https://doi.org/10.1007/s11046-009-9220-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-009-9220-x