Abstract
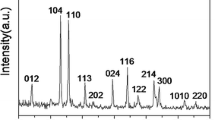
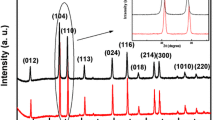
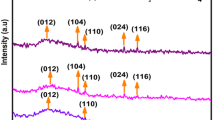
Polyhedron-shaped hematite (α-Fe2O3) nanoparticles have been successfully synthesized via a facile hydrothermal method by mixing FeCl3 and NH4OH at high temperature. In this work, the influences of experimental conditions such as the effects of the concentration of iron’s ion, NH4OH concentration, and reaction temperature on the hematite’s particle size, dispersity, and growth rate were investigated. Results show that hematite nanoparticles with good crystallinity with the particle size of 100 nm could be obtained when the hydrothermal reaction was carried out with concentration of Fe3+ = 16 mM, NH4OH = 40 mM, reaction temperature = 120 °C, and reaction time = 24 h. In addition, this study investigates the hematite nanoparticle-formation mechanism with reaction time. It is observed that the formation of hematite nanoparticles are initiated by the formation of intermediate phase of goethite nanorods in the early stage of hydrothermal reaction, which further transform into hematite crystal as the reaction is progressed.










Similar content being viewed by others
References
Almeida T, Fay M, Zhu Y, Brown P (2010) Hydrothermal growth mechanism of α-Fe2O3 nanorods derived by near in situ analysis. Nanoscale 2:2390–2399
Barlow D, Baird J, Su C (2004) Theory of the von Weimarn rules governing the average size of crystals precipitated from a supersaturated. J Cryst Growth 264:417–423
Bbusca G, Rossi PF (1983) FT-IR spectroscopic and calorimetric characterization of the adsorbed forms of water on α-Fe2O3 obtained by thermal decomposition of α-FeOOH. Mater Chem Phys 9:561–570
Beruto D (1983) Effect of water vapor on the evolution of the micropores of the α-Fe2O3 produced by α-FeOOH decomposition “in vacuo”. Mater Chem Phys 8:233–242
Burleson D, Penn RL (2006) Two-step growth of goethite from ferrihydrite. Langmuir 22:402–409
Byrappa K, Adschiri T (2007) Hydrothermal technology for nanotechnology. Prog Cryst Growth Charact 53:117–166
Caudron E, Tfayli A, Monnier C, Manfait M, Prognon P, Pradeau D (2011) Identification of hematite particles in sealed glass containers for pharmaceutical uses by Raman microspectroscopy. J Pharm Biomed 54:866–868
Cherian C, Sundaramurthy J, Kalaivani M, Ragupathy P, Kumar P, Thavasi V, Reddy M, Sow C, Mhaisalkar S, Ramakrishna S, Chowdari B (2012) Electrospun α-Fe2O3 nanorods as a stable, high capacity anode material for Li-ion batteries. J Mater Chem 22:12198–12204
Chernyshova I, Hochella M Jr, Madden A (2007) Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys Chem Chem Phys 9:1736–1750
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses, 2nd edn. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Cudennec Y, Lecerf A (2006) The transformation of ferrihydrate into goethite or hematite, revisited. J Solid State Chem 179:716–722
Darezereshki E (2011) One-step synthesis of hematite (α-Fe2O3) nano-particles by direct thermal-decomposition of meghemite. Mater Lett 65:642–645
de Faria D, Silva S, de Oliveira M (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28:873–878
Diamandescu L, Mihaila-Tarabasanu D, Colagero S (1997) Mössbauer study of the solid phase transformation α-FeOOH → Fe2O3. Mater Chem Phys 48:170–173
Fan H, You G, Li Y, Zheng Z, Tan H, Shen Z, Tang S, Feng Y (2009) Shape-controlled synthesis of single-crystalline Fe2O3 hollow nanocrystals and their tunable optical properties. J Phys Chem C 113:9928–9935
Fischer WR, Schwertmann U (1975) The formation of hematite from amorphous iron (III) hydroxide. Clay Clay Miner 23:33–37
Froment F, Tournié A, Colomban P (2008) Raman identification of natural red to yellow pigments: ochre and iron-containing ores. J Raman Spectrosc 39:560–568
Hassanjani-Roshan A, Vaezi M, Shokuhfar A, Rajabali Z (2011) Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particuology 9:95–99
Itoh H, Sugimoto T (2003) Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J Colloid Interface Sci 265:283–295
Jia B, Gao L (2008) Growth of well-defined cubic hematite single crystals: oriented aggregation and Ostwald ripening. Cryst Growth Des 8:1372–1376
Jia B, Gao L, Sun J (2007) Synthesis of single crystalline hematite polyhedral nanorods via a facile hydrothermal process. J Am Ceram Soc 90:1315–1318
Khan GG, Sarkar D, Singh AK, Mandal K (2013) Enhanced band gap emission and ferromagnetism of Au nanoparticles decorated α-Fe2O3. RCS Adv 3:1722–1727
Kulkarni S, Lokhande C (2003) Structural, optical, electrical and dielectrical properties of electrosynthesized nanocrystalline iron oxide thin films. Mater Chem Phys 82:151–156
Lee M, Park B, Chin I, Choi H (2009) Polymer modified hematite nanoparticles for electrophoretic display. J Electroceram 23:474–477
Lin M, Tan HR, Tan JPY, Bai S (2013) Understanding the growth mechanism of α-Fe2O3 nanoparticles through a controlled shape transformation. J Phys Chem C 117:11242–11250
Lu B, Li P, Liu H, Zhao L, Wei Y (2012) Synthesis of hexagonal pyramidal columnar hematite particles by a two-step solution route and their characterization. Powder Technol 215–216:132–136
Navale S, Bandgar D, Nalage S, Khuspe G, Chougule M, Kolekar Y, Sen S, Patil V (2013) Synthesis of Fe2O3 nanoparticles for nitrogen dioxide gas sensing applications. Ceram Int 39:6453–6460
Pu Z, Cao M, Yang J, Huang K, Hu C (2006) Controlled synthesis and growth mechanism of hematite nanorhombohedra, nanorods and nanocubes. Nanotechnology 17:799–804
Sarkar D, Khan GG, Singh AK, Mandal K (2012) Enhanced electrical, optical, and magnetic properties in multifunctional ZnO/α-Fe2O3 semiconductor nanoheterostructure by heterojunction engineering. J Phys Chem C 116:23540–23546
Schwertmann U, Friedl J, Stanjek H (1999) From Fe(III) ions to ferrihydrate and then to hematite. J Colloid Interface Sci 209:215–223
Schwertmann U, Stanjek H, Becher HH (2004) Long-term in vitro transformation of 2-line ferrihydrate to goethite/hematite at 4, 10, 15 and 25°C. Clay Miner 39:433–438
Sha G, Wang T, Xiao J, Liang C (2004) A mild solvothermal route to α-Fe2O3 nanoparticles. Mater Res Bull 39:1917–1921
Tadić M, Čitaković N, Panjan M, Stojanović Z, Marković D (2011) Synthesis, morphology, microstructure and magnetic properties of hematite submicron particles. J Alloy Compd 509:7639–7644
Xu Y, Yang S, Zhang G, Sun Y, Gao D, Sun Y (2011) Uniform hematite α-Fe2O3 nanoparticles: morphologies, size-controlled hydrothermal synthesis and formation mechanism. Mater Lett 65:1911–1914
Yang S, Xu Y, Sun Y, Zhang G, Gao D (2012) Size-controlled synthesis, magnetic property, and photocatalytic property of uniform α-Fe2O3 nanoparticles via a facile additive-free hydrothermal route. Cryst Eng Comm 14:7915–7921
Zhu M, Wang Y, Meng D, Qin X, Diao G (2012) Hydrothermal synthesis of hematite nanoparticles and their electrochemical properties. J Phys Chem C 116:16276–16285
Acknowledgments
The authors gratefully acknowledge the support of the Department of Energy through the National Energy Technology Laboratory under contract number DE-FE0005979. The authors appreciate the aid of Liz Bustamante for her assistance in language editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalil, M., Yu, J., Liu, N. et al. Hydrothermal synthesis, characterization, and growth mechanism of hematite nanoparticles. J Nanopart Res 16, 2362 (2014). https://doi.org/10.1007/s11051-014-2362-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2362-x