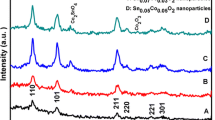
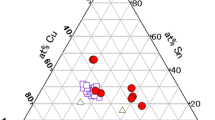
Abstract
The functional properties of quaternary I2–II–IV–VI4 nanomaterials, with potential interest in various technological fields, are highly sensitive to compositional variations, which is a challenging parameter to adjust. Here we demonstrate the presence of phosphonic acids to aid controlling the reactivity of the II element monomer to be incorporated in quaternary Cu2ZnSnSe4 nanoparticles and thus to provide a more reliable way to adjust the final nanoparticle metal ratios. Furthermore, we demonstrate the composition control in such multivalence nanoparticles to allow modifying charge carrier concentrations in nanomaterials produced from the assembly of these building blocks.



Similar content being viewed by others
References
Aldakov D, Lefrancois A, Reiss P (2013) Ternary and quaternary metal chalcogenide nanocrystals: synthesis properties and applications. J Mater Chem C 1:3756–3776
Berger LI, Prochukhan VD (1969) Ternary diamond-like semiconductors. Consultants Bureau, New York
Cao G, Lynch VM, Yacullo LN (1993) Synthesis, structural characterization, and intercalation chemistry of two layered cadmium organophosphonates. Chem Mater 5:1000–1006. doi:10.1021/cm00031a021
Carrete A et al (2013) Antimony-based ligand exchange to promote crystallization in spray-deposited Cu2ZnSnSe4 solar cells. J Am Chem Soc 135:15982–15985. doi:10.1021/ja4068639
Chen S, Gong XG, Walsh A, Wei S-H (2010) Defect physics of the kesterite thin-film solar cell absorber Cu2ZnSnS4. App Phys Lett 96:02. doi:10.1063/1.3275796
Dudchak IV, Piskach LV (2003) Phase equilibria in the Cu2SnSe3–SnSe2–ZnSe system. J Alloys Compd 351:145–150. doi:10.1016/S0925-8388
Fan F-J, Wang Y-X, Liu X-J, Wu L, Yu S-H (2012) Large-scale colloidal synthesis of Non-stoichiometric Cu2ZnSnSe4 nanocrystals for thermoelectric applications. Adv Mater 24:6158–6163. doi:10.1002/adma.201202860
Fella CM, Romanyuk YE, Tiwari AN (2013) Technological status of Cu2ZnSn(S, Se)4 thin film solar cells. Sol Energy Mater Sol Cells 119:276–277. doi:10.1016/j.solmat.2013.08.027
Fredoueil F, Evain M, Massiot D, Bujoli-Doeuff M, Janvier P, Clearfield A, Bujoli B (2002) Synthesis and characterization of two new cadmium phosphonocarboxylates Cd2(OH)(O3PC2H4CO2) and Cd3(O3PC2H4CO2)22H2O. J Chem Soc Dalton Trans 7:1508–1512. doi:10.1039/B110275N
García-Rodríguez R, Hendricks MP, Cossairt BM, Liu H, Owen JS (2013) Conversion reactions of cadmium chalcogenide nanocrystal precursors. Chem Mater 25:1233–1249. doi:10.1021/cm3035642
Guo Q, Hillhouse HW, Agrawal R (2009) Synthesis of Cu2ZnSnS4 nanocrystal ink and its use for solar cells. J Am Chem Soc 131:11672–11673
Haas W, Rath T, Pein A, Rattenberger J, Trimmel G, Hofer F (2011) The stoichiometry of single nanoparticles of copper zinc tin selenide. Chem Commun 47:2050–2052
Heinrich CP, Day TW, Zeier WG, Snyder GJ, Tremel W (2013) Effect of isovalent substitution on the thermoelectric properties of the Cu2ZnGeSe4–x S x series of solid solutions. J Am Chem Soc 136:442–448. doi:10.1021/ja410753k
Ibáñez M et al (2012a) Composition control and thermoelectric properties of quaternary chalcogenide nanocrystals: the case of stannite Cu2CdSnSe4. Chem Mater 24:562–570
Ibáñez M et al (2012b) Cu2ZnGeSe4 nanocrystals: synthesis and thermoelectric properties. J Am Chem Soc 134:4060–4063
Ibáñez M, Zamani R, Li W, Shavel A, Arbiol J, Morante JR, Cabot A (2012c) Extending the nanocrystal synthesis control to quaternary compositions. Cryst Growth Des 12:1085–1090
Ibáñez M et al (2013) Colloidal synthesis and thermoelectric properties of Cu2SnSe3 nanocrystals. J Mater Chem A 1:1421
Ikeda S, Nakamura T, Harada T, Matsumura M (2010) Multicomponent sulfides as narrow gap hydrogen evolution photocatalysts. Phys Chem Chem Phys 12:13943–13949. doi:10.1039/c0cp00267d
Ji X, Copenhaver D, Sichmeller C, Peng X (2008) Ligand bonding and dynamics on colloidal nanocrystals at room temperature: the case of alkylamines on CdSe nanocrystals. J Am Chem Soc 130:5726–5735. doi:10.1021/ja710909f
Khare A, Wills AW, Ammerman LM, Norris DJ, Aydil ES (2011) Size control and quantum confinement in Cu2ZnSnS4 nanocrystals. Chem Commun 47:11721–11723
LaLonde AD, Ikeda T, Snyder GJ (2011) Rapid consolidation of powdered materials by induction hot pressing. Rev Sci Instrum 82:025104. doi:10.1063/1.3534080
Li W et al (2013) Cu2HgSnSe4 nanoparticles: synthesis and thermoelectric properties. Cryst Eng Comm 15:8966–8971. doi:10.1039/c3ce41583j
Li W et al (2014) Colloidal synthesis and functional properties of quaternary Cu-Based semiconductors: Cu2HgGeSe4. J Nanopart Res 16:1–6. doi:10.1007/s11051-014-2297-2
Liu H, Owen JS, Alivisatos AP (2007) Mechanistic study of precursor evolution in colloidal Group II–VI semiconductor nanocrystal synthesis. J Am Chem Soc 129:305–312. doi:10.1021/ja0656696
Liu M-L, Chen IW, Huang F-Q, Chen L-D (2009a) Improved thermoelectric properties of Cu-doped quaternary chalcogenides of Cu2CdSnSe4. Adv Mater 21:3808–3812
Liu M-L, Huang F-Q, Chen L-D, Chen I-W (2009b) A wide-band-gap p-type thermoelectric material based on quaternary chalcogenides of Cu2ZnSnQ4 (Q=S, Se). Appl Phys Lett 94:202103
Mitzi DB, Gunawan O, Todorov TK, Wang K, Guha S (2011) The path towards a high-performance solution-processed kesterite solar cell. Sol Energ Mat Sol C 95:1421–1436
Miyauchi M, Hanayama T, Atarashi D, Sakai E (2012) Photoenergy conversion in p-Type Cu2ZnSnS4 nanorods and n-Type metal oxide composites. J Phys Chem C 116:23945–23950. doi:10.1021/jp307949n
Nakamura S, Maeda T, Wada T (2010) Phase stability and electronic structure of In-free photovoltaic materials: Cu2ZnSiSe4, Cu2ZnGeSe4, and Cu2ZnSnSe4. Jpn J Appl Phys 49:121203
Peng ZA, Peng X (2002) Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes: nucleation and growth. J Am Chem Soc 124:3343–3353. doi:10.1021/ja0173167
Pradhan N, Reifsnyder D, Xie R, Aldana J, Peng X (2007) Surface ligand dynamics in growth of nanocrystals. J Am Soc Chem 129:9500–9509. doi:10.1021/ja0725089
Riha SC, Parkinson BA, Prieto AL (2009) Solution-based synthesis and characterization of Cu2ZnSnS4 nanocrystals. J Am Chem Soc 131:12054–12055
Riha SC, Parkinson BA, Prieto AL (2011) Compositionally tunable Cu2ZnSn(S(1−x)Se(x))4 Nanocrystals: probing the effect of Se-inclusion in mixed chalcogenide thin films. J Am Chem Soc 133:15272–15275
Sevik C, Cagin T (2009) Assessment of thermoelectric performance of Cu2ZnSnX4, X=S, Se, and Te. Appl Phys Lett 95:112105
Shavel A, Arbiol J, Cabot A (2010) Synthesis of quaternary chalcogenide nanocrystals: stannite Cu2Zn x Sn y Se1+x+2y . J Am Chem Soc 132:4514–4515
Singh A, Geaney H, Laffir F, Ryan KM (2012) Colloidal synthesis of wurtzite Cu2ZnSnS4 nanorods and their perpendicular assembly. J Am Chem Soc 134:2910–2913
Singh A, Singh S, Levcenko S, Unold T, Laffir F, Ryan KM (2013) Compositionally tunable photoluminescence emission in Cu2ZnSn(S1−x Se x )4 nanocrystals. Angew Chem Int Ed 52:9120–9124. doi:10.1002/anie.201302867
Tanaka K, Fukui Y, Moritake N, Uchiki H (2011) Chemical composition dependence of morphological and optical properties of Cu2ZnSnS4 thin films deposited by sol–gel sulfurization and Cu2ZnSnS4 thin film solar cell efficiency. Sol Energ Mat Sol C 95:838–842
Todorov TK, Tang J, Bag S, Gunawan O, Gokmen T, Zhu Y, Mitzi DB (2012) Beyond 11 % efficiency: characteristics of state-of-the-art Cu2ZnSn(S, Se)4. Solar Cells Adv Energy Mater 3(1):34–38. doi:10.1002/aenm.201200348
Tsuyoshi M, Satoshi N, Takahiro W (2011) First principles calculations of defect formation in In-free photovoltaic semiconductors Cu 2 ZnSnS 4 and Cu 2 ZnSnSe4. Jpn J Appl Phys 50(4S):04DP07
Wang W, Banerjee S, Jia S, Steigerwald ML, Herman IP (2007) Ligand control of growth morphology and capping structure of colloidal CdSe nanorods. Chem Mater 19:2573–2580. doi:10.1021/cm0705791
Xiao W et al (2015) Intrinsic defects and Na doping in Cu2ZnSnS4: a density-functional theory study. Sol Energy 116:125–132. doi:10.1016/j.solener.2015.04.005
Yu X, Shavel A, An X, Luo Z, Ibáñez M, Cabot A (2014) Cu2ZnSnS4–Pt and Cu2ZnSnS4–Au heterostructured nanoparticles for photocatalytic water splitting and pollutant degradation. J Am Chem Soc 136:9236–9239. doi:10.1021/ja502076b
Yu X, An X, Genç A, Ibáñez M, Arbiol J, Zhang Y, Cabot A (2015a) Cu2ZnSnS4–PtM (M=Co, Ni) nanoheterostructures for photocatalytic hydrogen evolution. J Phys Chem C 119:21882–21888. doi:10.1021/acs.jpcc.5b06199
Yu X et al (2015b) Cu2ZnSnS4–Ag2S nanoscale p–n Heterostructures as sensitizers for photoelectrochemical water splitting. Langmuir 31:10555–10561. doi:10.1021/acs.langmuir.5b02490
Zeier WG, LaLonde A, Gibbs ZM, Heinrich CP, Panthöfer M, Snyder GJ, Tremel W (2012) Influence of a nano phase segregation on the thermoelectric properties of the p-Type doped stannite compound Cu2+x Zn1−x GeSe4. J Am Chem Soc 134:7147–7154. doi:10.1021/ja301452j
Acknowledgments
At IREC, work was supported by European Regional Development Funds and the Framework 7 program under project UNION (FP7-NMP 310250). M.I. Thanks AGAUR for her Beatriu i Pinós post-doctoral Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibáñez, M., Berestok, T., Dobrozhan, O. et al. Phosphonic acids aid composition adjustment in the synthesis of Cu2+x Zn1−x SnSe4−y nanoparticles. J Nanopart Res 18, 226 (2016). https://doi.org/10.1007/s11051-016-3545-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3545-4