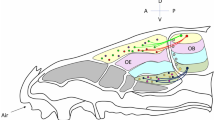
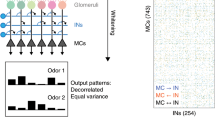
A possible mechanism that provides increased selectivity of olfactory bulb projection neurons, as compared to that of the primary olfactory receptor neurons, has been proposed. The mechanism operates at low concentrations of the odor molecules, when the lateral inhibition mechanism becomes inefficient. The mechanism proposed is based on a threshold-type reaction to the stimuli received by a projection neuron from a few receptor neurons, the stochastic nature of these stimuli, and the existence of electrical leakage in the projection neurons. The mechanism operates at the level of the single individual projection neuron and does not require the involvement of other bulbar neurons.
Similar content being viewed by others
References
K. J. Ressler, S. L. Sullivan, and L. B. Buck, “Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb,” Cell, 79, 1245–1255 (1994).
V. W. Drongelen, “Unitary recordings of near threshold responses of receptor cells in the olfactory mucosa of the frog,” J. Physiol., 277, No. 1, 423–435 (1978).
V. W. Drongelen, A. Holley, and K. B. Døving, “Convergence in the olfactory system: Quantitative aspects of odour sensitivity,” J. Theor. Biol., 71, No. 1, 39–48 (1978).
P. Duchamp-Viret, A. Duchamp, and M. Vigoroux, “Amplifying role of convergence in olfactory system. A comparative study of receptor cell and second-order neuron sensitivities,” J. Neurophysiol., 61, No. 5, 1085–1094 (1989).
A. Duchamp, “Electrophysiological responses of olfactory bulb neurons to odour stimuli in the frog. A comparison with receptor cells,” Chem. Senses,7, No. 2, 191–210 (1982).
S. Kikuta, M. L. Fletcher, R. Homma et al., “Odorant response properties of individual neurons in an olfactory glomerular module,” Neuron, 77, No. 6, 1122–1135 (2013).
A. P. Davison, J. Feng, and D. Brown, “Dendrodendritic inhibition and simulated odor responses in a detailed olfactory bulb network model,” J. Neurophysiol., 90, No. 3, 1921–1935 (2003).
T. A. Cleland and C. Linster, “Computation in the olfactory system,” Chem. Senses, 30, No. 9, 801–813 (2005).
R. Granit and J. C. Eccles, “Aspects of excitation and inhibition in the retina,” Proc. Roy. Soc. Lond. Ser. B Biol. Sci.,140, No. 899, 191–199 (1952).
H. B. Barlow, “Summation and inhibition in the frog’s retina,” J. Physiol.,119, No. 1, 69–88 (1953).
H. K. Hartline, H. G. Wagner, and F. Ratliff, “Inhibition in the eye of Limulus,” J. Gen. Physiol., 39, No. 5, 651–673 (1956).
M. Yokoi, K. Mori, and S. Nakanishi, “Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb,” Proc. Natl. Acad. Sci. USA,92, No. 8, 3371–3375 (1995).
N. N. Urban and B. Sakmann, “Reciprocal intra glomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells,” J. Physiol., 542, No. 2, 355–367 (2002).
A. L. Fantana, E. R. Soucy, and M. Meister, “Rat olfactory bulb mitral cells receive sparse glomerular inputs,” Neuron,59, No. 5, 802–814 (2008).
M. T. Valley and S. Firestein, “A lateral look at olfactory bulb lateral inhibition,” Neuron,59, No. 5, 682–684 (2008).
P. Duchamp-Viret, A. Duchamp, and G. Sicard, “Olfac tory discrimination over a wide concentration range. Compa rison of receptor cell and bulb neuron abilities,” Brain Res., 517, Nos. 1–2, 256-262 (1990).
A. K. Vidybida, “Selectivity of chemoreceptor neuron,” BioSystems,58, 125–132 (2000).
A. K. Vidybida, A. S. Usenko, and J. P. Rospars, “Selectivity improvement in a model of olfactory receptor neuron with adsorption-desorption noise,” J. Biol. Syst., 16, No. 4, 531–545 (2008).
A. K. Vidybida, “Adsorption–desorption noise can be used for improving selectivity,” Sensors Actuators A:Physical., 107, No. 3, 233–237 (2003).
V. S. Korolyuk, P. G. Kostyuk, B. Ya. Pjatigorskii, and E. P. Tkachenko, “Mathematical model of spontaneous activity of some neurons in the CNS,” Biofizika,12, No. 5, 895–899 (1967).
L. F. Abbott, “Lapique’s introduction of the integrateand- fire model neuron (1907),” Brain Res. Bull., 50, Nos. 5/6, 303–304 (1999).
L. B. Buck, “The molecular architecture of odor and pheromone sensing in mammals,” Cell,100, No. 6, 611–618 (2000).
A. K. Vidybida, Stochastic Models, NAS of Ukraine, BITP, Kyiv (2006).
J. N. Bourne and N. E. Schoppa, “Three-dimensional synaptic analyses of mitral cell and external tufted cell dendrites in rat olfactory bulb glomeruli,” J. Comp. Neurol., 525, No. 3, 592–609 (2017).
S. D. Burton and N. N. Urban, “Greater excitability and firing irregularity of tufted cells underlies distinct afferent-evoked activity of olfactory bulb mitral and tufted cells,” J. Physiol., 592, No. 10, 2097–2118 (2014).
J. Tan, A. Savigner, M. Ma, and M. Luo, “Odor information processing by the olfactory bulb analyzed in gene-targeted mice,” Neuron,65, No. 6, 912–926 (2010).
K. Mori, M. C. Nowycky, and G. M. Shepherd, “Electrophysiological analysis of mitral cells in the isolated turtle olfactory bulb,” J. Physiol., 314, No. 1, 281–294 (1981).
R. J. Sayer, M. J. Friedlander, and S. J. Redman, “The time course and amplitude of EPSPs evoked at synapses between pairs of CA3/CA1 neurons in the hippocampal slice,” J. Neurosci., 10, No. 3, 826–836 (1990).
A. Duchamp and G. Sicard, “Influence of stimulus intensity on odour discrimination by olfactory bulb neurons as compared with receptor cells,” Chem. Senses,8, No. 4, 355–366 (1984).
P. Duchamp-Viret, and A. Duchamp, “Odor processing in the frog olfactory system,” Prog. Neurobiol., 53, No. 5, 561–602 (1997).
G. Lowe and G. H. Gold, “Olfactory transduction is intrinsically noisy,” Proc. Natl. Acad. Sci. USA, 92, No. 17, 7864–7868 (1995).
J. P. Rospars, P. Lánský, J. Vaillant, et al., “Spontaneous activity of first- and second-order neurons in the frog olfactory system,” Brain Res.,662, Nos. 1–2, 31–44 (1994).
V. Bhandawat, G. Maimon, M. H. Dickinson, and R. J. Wilson, “Olfactory modulation of flight in Drosophila is sensitive, selective and rapid,” J. Exp. Biol., 213, No. 21, 3625 (2010).
M. Häusser, N. Spruston, and G. J. Stuart, “Diversity and dynamics of dendritic signaling,” Science,290, No. 5492, 739–744 (2000).
M. London and M. Häusser, “Dendritic computation,” Ann. Rev. Neurosci., 28, No. 1, 503–532 (2005).
J. P. Rospars, A. Grémiaux, D. Jarriault, et al., “Heterogeneity and convergence of olfactory first-order neurons account for the high speed and sensitivity of secondorder neurons,” PLOS Comput. Biol., 10, No. 12 (2014): e1003975.
J. P. McGann. Presynaptic inhibition of olfactory sensory neurons: New mechanisms and potential functions,” Chem. Senses,38, No. 6, 459–474 (2013).
P. M. Lledo, G. Gheusi, and J. D. Vincent, “Information processing in the mammalian olfactory system,” Physiol. Rev., 85, No. 1, 281–317 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vidybida, A.K. Possible Stochastic Mechanism for Improving the Selectivity of Olfactory Projection Neurons. Neurophysiology 51, 152–159 (2019). https://doi.org/10.1007/s11062-019-09808-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-019-09808-6