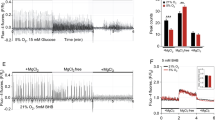
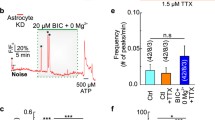
Abstract
A ketogenic diet (KD; high-fat, low-carbohydrate) can benefit refractory epilepsy, but underlying mechanisms are unknown. We used mice inducibly expressing a mutated form of the mitochondrial DNA repair enzyme UNG1 (mutUNG1) to cause progressive mitochondrial dysfunction selectively in forebrain neurons. We examined the levels of mRNAs and proteins crucial for mitochondrial biogenesis and dynamics. We show that hippocampal pyramidal neurons in mutUNG1 mice, as well as cultured rat hippocampal neurons and human fibroblasts with H2O2 induced oxidative stress, improve markers of mitochondrial biogenesis, dynamics and function when fed on a KD, and when exposed to the ketone body β-hydroxybutyrate, respectively, by upregulating PGC1α, SIRT3 and UCP2, and (in cultured cells) increasing the oxygen consumption rate (OCR) and the NAD+/NADH ratio. The mitochondrial level of UCP2 was significantly higher in the perikarya and axon terminals of hippocampus CA1 pyramidal neurons in KD treated mutUNG1 mice compared with mutUNG1 mice fed a standard diet. The β-hydroxybutyrate receptor GPR109a (HCAR2), but not the structurally closely related lactate receptor GPR81 (HCAR1), was upregulated in mutUNG1 mice on a KD, suggesting a selective influence of KD on ketone body receptor mechanisms. We conclude that progressive mitochondrial dysfunction in mutUNG1 expressing mice causes oxidative stress, and that exposure of animals to KD, or of cells to ketone body in vitro, elicits compensatory mechanisms acting to augment mitochondrial mass and bioenergetics via the PGC1α-SIRT3-UCP2 axis (The compensatory processes are overwhelmed in the mutUNG1 mice by all the newly formed mitochondria being dysfunctional).





Similar content being viewed by others
References
Kann O, Kovacs R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292:C641–C657
Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA 105:7070–7075
Richter C, Park JW, Ames BN (1988) Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA 85:6465–6467
Larsen NB, Rasmussen M, Rasmussen LJ (2005) Nuclear and mitochondrial DNA repair: similar pathways? Mitochondrion 5:89–108
Wallace DC (1994) Mitochondrial DNA sequence variation in human evolution and disease. Proc Natl Acad Sci USA 91:8739–8746
Anderson CT, Friedberg EC (1980) The presence of nuclear and mitochondrial uracil-DNA glycosylase in extracts of human KB cells. Nucleic Acids Res 8:875–888
Cervenka MC, Henry B, Nathan J, Wood S, Volek JS (2013) Worldwide dietary therapies for adults with epilepsy and other disorders. J Child Neurol 28:1034–1040
Kessler SK, Neal EG, Camfield CS, Kossoff EH (2011) Dietary therapies for epilepsy: future research. Epilepsy Behav 22:17–22
Kossoff EH, Zupec-Kania BA, Rho JM (2009) Ketogenic diets: an update for child neurologists. J Child Neurol 24:979–988
Levy R, Cooper P (2003) Ketogenic diet for epilepsy. Cochrane Database Syst Rev 2003:CD001903
Levy RG, Cooper PN, Giri P (2012) Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001903.pub2
Villeneuve N, Pinton F, Bahi-Buisson N, Dulac O, Chiron C, Nabbout R (2009) The ketogenic diet improves recently worsened focal epilepsy. Dev Med Child Neurol 51:276–281
Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M Jr, Boison D, Masino SA (2013) Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS ONE 8:e65021
Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM (2006) A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci 7:29
Ruskin DN, Ross JL, Kawamura M Jr, Ruiz TL, Geiger JD, Masino SA (2011) A ketogenic diet delays weight loss and does not impair working memory or motor function in the R6/2 1J mouse model of Huntington’s disease. Physiol Behav 103:501–507
Van der Auwera I, Wera S, Van Leuven F, Henderson ST (2005) A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab 2:28
Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB (2005) Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology 64:728–730
Hartman AL, Vining EP (2007) Clinical aspects of the ketogenic diet. Epilepsia 48:31–42
Nordi DR, De Vivo DC (2004) Effects of the ketogenic diet on cerebral energy metabolism. In: Stafstrom CE, Rho JM (eds) Epilepsy and the ketogenic diet, 1st edn. Humana Press, Totowa, pp 179–184
Yudkoff M, Daikhin Y, Nissim I, Nissim I (2004) The ketogenic diet: interactions with brain amino acid handling. In: Stafstrom CE, Rho JM (eds) Epilepsy and the ketogenic diet, 1st edn. Humana Press, Totowa, pp 185–200
Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, Verdin E (2017) Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab 26:547–557.e548
Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA (2017) A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 26:539–546.e535
Halestrap AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447:619–628
Forero-Quintero LS, Deitmer JW, Becker HM (2017) Reduction of epileptiform activity in ketogenic mice: the role of monocarboxylate transporters. Sci Rep 7:4900
Nedergaard J, Cannon B (2003) The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol 88:65–84
Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM (2004) The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol 55:576–580
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18
Pecqueur C, Alves-Guerra MC, Gelly C, Levi-Meyrueis C, Couplan E, Collins S, Ricquier D, Bouillaud F, Miroux B (2001) Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem 276:8705–8712
Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL (2003) Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinology 144:5014–5021
Toda C, Diano S (2014) Mitochondrial UCP2 in the central regulation of metabolism. Best Pract Res Clin Endocrinol Metab 28:757–764
Bechmann I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL (2002) Brain mitochondrial uncoupling protein 2 (UCP2): a protective stress signal in neuronal injury. Biochem Pharmacol 64:363–367
Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T (2006) Transgenic mice with a reduced core body temperature have an increased life span. Science 314:825–828
Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL (2005) Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab 1:145–152
Hirose M, Schilf P, Lange F, Mayer J, Reichart G, Maity P, Johren O, Schwaninger M, Scharffetter-Kochanek K, Sina C, Sadik CD, Kohling R, Miroux B, Ibrahim SM (2016) Uncoupling protein 2 protects mice from aging. Mitochondrion 30:42–50
Andrews ZB, Horvath B, Barnstable CJ, Elsworth J, Yang L, Beal MF, Roth RH, Matthews RT, Horvath TL (2005) Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson’s disease. J Neurosci 25:184–191
Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, Bartfai T (2005) Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem 93:493–501
Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T (2003) Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med 9:1062–1068
Bough K (2008) Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia 49(Suppl 8):91–93
Srivastava S, Baxa U, Niu G, Chen X, Veech RL (2013) A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 65:58–66
Hutfles LJ, Wilkins HM, Koppel SJ, Weidling IW, Selfridge JE, Tan E, Thyfault JP, Slawson C, Fenton AW, Zhu H, Swerdlow RH (2017) A bioenergetics systems evaluation of ketogenic diet liver effects. Appl Physiol Nutr Metab 42:955–962
Yin J, Han P, Tang Z, Liu Q, Shi J (2015) Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab 35:1783–1789
Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW (2013) Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci USA 110:6601–6606
Pagel-Langenickel I, Bao J, Joseph JJ, Schwartz DR, Mantell BS, Xu X, Raghavachari N, Sack MN (2008) PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J Biol Chem 283:22464–22472
Lomb DJ, Laurent G, Haigis MC (2010) Sirtuins regulate key aspects of lipid metabolism. Biochim Biophys Acta 1804:1652–1657
Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH (2017) Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 16:4–16
Su J, Liu J, Yan XY, Zhang Y, Zhang JJ, Zhang LC, Sun LK (2017) Cytoprotective effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia-reperfusion injury. Int J Mol Sci 18
Lauritzen KH, Moldestad O, Eide L, Carlsen H, Nesse G, Storm JF, Mansuy IM, Bergersen LH, Klungland A (2010) Mitochondrial DNA toxicity in forebrain neurons causes apoptosis, neurodegeneration, and impaired behavior. Mol Cell Biol 30:1357–1367
Dianov GL, Sleeth KM, Dianova II, Allinson SL (2003) Repair of abasic sites in DNA. Mutat Res 531:157–163
Pinz KG, Shibutani S, Bogenhagen DF (1995) Action of mitochondrial DNA polymerase gamma at sites of base loss or oxidative damage. J Biol Chem 270:9202–9206
Lauritzen KH, Dalhus B, Storm JF, Bjoras M, Klungland A (2011) Modeling the impact of mitochondrial DNA damage in forebrain neurons and beyond. Mech Ageing Dev 132:424–428
Loeb LA, Wallace DC, Martin GM (2005) The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci USA 102:18769–18770
Nicholls DG (2008) Oxidative stress and energy crises in neuronal dysfunction. Ann NY Acad Sci 1147:53–60
Lauritzen KH, Hasan-Olive MM, Regnell CE, Kleppa L, Scheibye-Knudsen M, Gjedde A, Klungland A, Bohr VA, Storm-Mathisen J, Bergersen LH (2016) A ketogenic diet accelerates neurodegeneration in mice with induced mitochondrial DNA toxicity in the forebrain. Neurobiol Aging 48:34–47
Bergersen LH, Storm-Mathisen J, Gundersen V (2008) Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc 3:144–152
Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP (2016) 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem 139:769–781
Reddy PH, Shirendeb UP (2012) Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim Biophys Acta 1822:101–110
Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou MH (2011) Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS ONE 6:e25436
Coyle CH, Kader KN (2007) Mechanisms of H2O2-induced oxidative stress in endothelial cells exposed to physiologic shear stress. ASAJO J 53:17–22
Olichon A, Guillou E, Delettre C, Landes T, Arnaune-Pelloquin L, Emorine LJ, Mils V, Daloyau M, Hamel C, Amati-Bonneau P, Bonneau D, Reynier P, Lenaers G, Belenguer P (2006) Mitochondrial dynamics and disease, OPA1. Biochim Biophys Acta 1763:500–509
Lu B (2009) Mitochondrial dynamics and neurodegeneration. Curr Neurol Neurosci Rep 9:212–219
Kuei C, Yu J, Zhu J, Wu J, Zhang L, Shih A, Mirzadegan T, Lovenberg T, Liu C (2011) Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol Pharmacol 80:848–858
Richard D, Clavel S, Huang Q, Sanchis D, Ricquier D (2001) Uncoupling protein 2 in the brain: distribution and function. Biochem Soc Trans 29:812–817
Simuni T, Sethi K (2008) Nonmotor manifestations of Parkinson’s disease. Ann Neurol 64(Suppl 2):S65–S80
Ahola-Erkkila S, Carroll CJ, Peltola-Mjosund K, Tulkki V, Mattila I, Seppanen-Laakso T, Oresic M, Tyynismaa H, Suomalainen A (2010) Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Genet 19:1974–1984
Al-Zaid NS, Dashti HM, Mathew TC, Juggi JS (2007) Low carbohydrate ketogenic diet enhances cardiac tolerance to global ischaemia. Acta Cardiol 62:381–389
Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ (2006) Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol 60:223–235
Kim DK, Heineman FW, Balaban RS (1991) Effects of beta-hydroxybutyrate on oxidative metabolism and phosphorylation potential in canine heart in vivo. Am J Physiol 260:H1767–H1773
Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S (2003) D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112:892–901
Sampson M, Lathen D, Dallon B, Draney C, Ray J, Kener K, Parker B, Witt J, Gibbs J, Tessem JS, Bikman BT (2017) Beta-hydroxybutyrate favorably alters b-cell survival and mitochondrial bioenergetics. FASEB J 31:883.888
Masuda R, Monahan JW, Kashiwaya Y (2005) D-beta-hydroxybutyrate is neuroprotective against hypoxia in serum-free hippocampal primary cultures. J Neurosci Res 80:501–509
Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, Zhang D, Zhang XM, Samuel VT, Shulman GI (2010) A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab 299:E808–E815
Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79–99
Chen H, Chan DC (2006) Critical dependence of neurons on mitochondrial dynamics. Curr Opin Cell Biol 18:453–459
Brenmoehl J, Hoeflich A (2013) Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 13:755–761
Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, Jacobson MP, Verdin E (2010) SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab 12:654–661
Liu J, Li D, Zhang T, Tong Q, Ye RD, Lin L (2017) SIRT3 protects hepatocytes from oxidative injury by enhancing ROS scavenging and mitochondrial integrity. Cell Death Dis 8:e3158
Ding Y, Yang H, Wang Y, Chen J, Ji Z, Sun H (2017) Sirtuin 3 is required for osteogenic differentiation through maintenance of PGC-1a-SOD2-mediated regulation of mitochondrial function. Int J Biol Sci 13:254–264
Elamin M, Ruskin DN, Masino SA, Sacchetti P (2017) Ketone-based metabolic therapy: is increased NAD(+) a primary mechanism? Front Mol Neurosci 10:377
Acknowledgements
This study was supported by research Grants from the Research Council of Norway, the Norwegian Health Association, and the Lundbeck Foundation (Denmark).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no actual or potential conflicts of interest. All aspects of the research described here were carried out in accordance with the laws of Norway concerning animal research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hasan-Olive, M.M., Lauritzen, K.H., Ali, M. et al. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem Res 44, 22–37 (2019). https://doi.org/10.1007/s11064-018-2588-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2588-6