Abstract
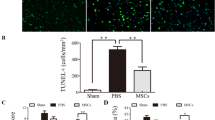
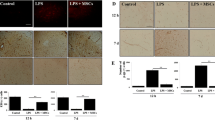
To investigate the influence of tumor necrosis factor-stimulated gene-6 (TSG-6) secreted by bone mesenchymal stem cells (BMSCs) on blood brain barrier (BBB) after intracerebral hemorrhage (ICH) and its related mechanisms. BMSCs and astrocytes were isolated and induced by TNF-α and LPS respectively. The effect of TSG-6 secreted by BMSCs on the proliferation and apoptosis of astrocytes and inflammatory response were assessed by CCK8, flow cytometry, and ELISA respectively. Then we studied the effects of TSG-6 secreted by BMSCs through the paracrine mechanism on the integrity of BBB after ICH via NF-κB signaling pathway in vitro and in vivo. We successfully isolated BMSCs and astrocytes. After LPS treatment of astrocytes, IL-1β, IL-6, and TNF-α showed an upward trend. TSG-6 secreted by TNF-α-activated BMSCs could antagonize the inflammatory response in activated astrocytes. Through the co-culture of astrocytes and BMSCs and the ICH animal model, we found that TSG-6 regulates activated astrocytes by inhibiting the NF-κB signaling pathway and ameliorates BBB damage. Furthermore, we found that TNF-α-activated BMSCs secreted exosomes containing TSG-6 and played an anti-inflammatory effect. TSG-6 secreted by BMSCs regulates activated astrocytes by inhibiting the NF-κB signaling pathway, thereby ameliorating BBB damage.








Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Hemphill JC et al (2018) Clinical performance measures for adults hospitalized with intracerebral hemorrhage: performance measures for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(7):e243–e261. https://doi.org/10.1161/STR.0000000000000171
Xi T et al (2017) MicroRNA-126-3p attenuates blood–brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem Biophys Res Commun 494(1–2):144–151
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201
Aragon MJ et al (2017) Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood–brain barrier impairment. Proc Natl Acad Sci USA 114(10):1968–1976
Abbott NJ (2002) Astrocyte-endothelial interactions and blood–brain barrier permeability. J Anat 200(6):629–638
Gimble JM et al (2010) Adipogenesis in a myeloid supporting bone marrow stromal cell line. J Cell Biochem 50(1):73–82
Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276(5309):71–74
Tang G et al (2014) Mesenchymal stem cells maintain blood–brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells 32(12):3150–3162
Lee RH et al (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5(1):54–63
Liu L et al (2016) TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci Rep 6:30121
Song WJ et al (2017) TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates dss-induced colitis by inducing M2 macrophage polarization in mice. Sci Rep 7(1):5187
Xin H et al (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33(11):1711–1715
Shen H et al (2018) Role of exosomes derived from miR-133b modified MSCs in an experimental rat model of intracerebral hemorrhage. J Mol Neurosci 64(3):421–430
Yuxia H et al (2018) Multipotent mesenchymal stromal cell–derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J Neurosurg 131(1):290–300
Wagner KR (2007) Modeling intracerebral hemorrhage: glutamate, nuclear factor-kappa B signaling and cytokines. Stroke 38(2 Suppl):753–758
Shen X et al (2016) Autophagy regulates intracerebral hemorrhage induced neural damage via apoptosis and NF-κB pathway. Neurochem Int 96:100–112
Liu DL et al (2016) Peroxiredoxin 1-mediated activation of TLR4/NF-κB pathway contributes to neuroinflammatory injury in intracerebral hemorrhage. Int Immunopharmacol 41:82–89
Gong P et al (2019) Novel insights into MSK1 phosphorylation by MRKβ in intracerebral hemorrhage-induced neuronal apoptosis. Cell Transplant 28(6):783–795
Chang CF et al (2020) Bexarotene enhances macrophage erythrophagocytosis and hematoma clearance in experimental intracerebral hemorrhage. Stroke 51(2):612–618
Gong Y et al (2019) Establishment of an experimental intracerebral haemorrhage model for mass effect research using a thermo-sensitive hydrogel. Sci Rep 9(1):13838
Schlunk F, Greenberg SM (2015) The pathophysiology of intracerebral hemorrhage formation and expansion. Transl Stroke Res 6(4):257–263
Shi Y et al (2016) Translational stroke research on blood–brain barrier damage: challenges, perspectives, and goals. Transl Stroke Res 7(2):89–92
Choi H et al (2011) Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 118(2):330–338
Chen M et al (2015) The inhibitory effect of mesenchymal stem cell on blood–brain barrier disruption following intracerebral hemorrhage in rats: contribution of TSG-6. J Neuroinflammation 12:61
Zhao C et al (2007) TNF-alpha knockout and minocycline treatment attenuates blood–brain barrier leakage in MPTP-treated mice. Neurobiol Dis 26(1):36–46
Ryu JK, McLarnon JG (2006) Minocycline or iNOS inhibition block 3-nitrotyrosine increases and blood–brain barrier leakiness in amyloid beta-peptide-injected rat hippocampus. Exp Neurol 198(2):552–557
Zhu Q et al (2018) Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-κB pathway after subarachnoid hemorrhage in rats. J Neuroinflammation 15(1):178
Shang Y et al (2019) MicroRNA-93 regulates the neurological function, cerebral edema and neuronal apoptosis of rats with intracerebral hemorrhage through TLR4/NF-κB signaling pathway. Cell Cycle 18(22):3160–3176
Kortekaas R et al (2005) Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57(2):176–179
Jin MS et al (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130(6):1071–1082
Zhu H et al (2018) TLR2 ligand Pam3CSK4 regulates MMP-2/9 expression by MAPK/NF-κB signaling pathways in primary brain microvascular endothelial cells. Neurochem Res 43(10):1897–1904
Bolton C et al (2009) A comparative evaluation of the response to peroxynitrite by a brain endothelial cell line and control of the effects by drug targeting. Cell Mol Neurobiol 29(5):707–717
Torreilles F et al (1999) Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev 30(2):153–163
Stamatovic SM et al (2016) Junctional proteins of the blood–brain barrier: new insights into function and dysfunction. Tissue Barriers 4(1):e1154641
Mohammadi MT (2016) Overproduction of nitric oxide intensifies brain infarction and cerebrovascular damage through reduction of claudin-5 and ZO-1 expression in striatum of ischemic brain. Pathol Res Pract 212(11):959–964
Camire RB et al (2014) Biphasic modulation of paracellular claudin-5 expression in mouse brain endothelial cells is mediated through the phosphoinositide-3-kinase/AKT pathway. J Pharmacol Exp Ther 351(3):654–662
Gürsoy-Ozdemir Y, Can A, Dalkara T (2004) Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 35(6):1449–1453
Bolton SJ, Anthony DC, Perry VH (1998) Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood–brain barrier breakdown in vivo. Neuroscience 86(4):1245–1257
Lee PH et al (2012) A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol 72(1):32–40
Jiang L et al (2020) Exosomes derived from TSG-6 modified mesenchymal stromal cells attenuate scar formation during wound healing. Biochimie 177:40–49
Chaubey S et al (2018) Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther 9(1):173
Bertani B, Ruiz N (2018) Function and biogenesis of lipopolysaccharides. EcoSal Plus. https://doi.org/10.1128/ecosalplus.ESP-0001-2018
Fei X et al (2019) The role of Toll-like receptor 4 in apoptosis of brain tissue after induction of intracerebral hemorrhage. J Neuroinflammation 16(1):234
Zhang F, Zhang C (2018) Rnf112 deletion protects brain against intracerebral hemorrhage (ICH) in mice by inhibiting TLR-4/NF-κB pathway. Biochem Biophys Res Commun 507(1–4):43–50
Zhou L et al (2020) DHZCP modulates microglial M1/M2 polarization via the p38 and TLR4/NF-κB signaling pathways in LPS-stimulated microglial cells. Front Pharmacol 11:1126
Natarajan R, Northrop N, Yamamoto B (2017) Fluorescein isothiocyanate (FITC)-dextran extravasation as a measure of blood–brain barrier permeability. Curr Protoc Neurosci 79:9.58.1-9.58.15
Khamchai S et al (2020) Morin protects the blood–brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci Rep 10(1):13379
Kim Y et al (2020) CLEC14A deficiency exacerbates neuronal loss by increasing blood–brain barrier permeability and inflammation. J Neuroinflammation 17(1):48
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos.81760224) and Youth Foundation of the Second Affiliated Hospital of Nanchang University (2019YNQN12014).
Author information
Authors and Affiliations
Contributions
BT performed the experiment, analyzed the data, and wrote the original draft; MS, XX, DL, QT and XW performed the experiment; MC guided the experiment, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
This study was approved by the animal experiment ethics committee of the First Affiliated Hospital of Nanchang University and the Second Affiliated Hospital of Nanchang University and conducted in strict accordance with the national institutes of health guidelines for the care and use of experimental animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, B., Song, M., Xie, X. et al. Tumor Necrosis Factor-stimulated Gene-6 (TSG-6) Secreted by BMSCs Regulates Activated Astrocytes by Inhibiting NF-κB Signaling Pathway to Ameliorate Blood Brain Barrier Damage After Intracerebral Hemorrhage. Neurochem Res 46, 2387–2402 (2021). https://doi.org/10.1007/s11064-021-03375-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03375-1