Abstract
At some point in life’s development, membranes formed, providing barriers between the environment and the interior of the ‘cell.’ This paper evaluates the research to date on the prebiotic origin of cell membranes and highlights possible areas of continuing study. A careful review of the literature uncovered unexpected factors that influence membrane evolution. The major stages in primitive membrane formation and the transition to contemporary cell membranes appear to require an exacting relationship between environmental conditions and amphiphile composition and phase behavior. Also, environmental and compositional requirements for individual stages are in some instances incompatible with one another, potentially stultifying the pathway to contemporary membranes. Previous studies in membrane evolution have noted the effects composition and environment have on membrane formation but the crucial dependence and interdependence on these two factors has not been emphasized. This review makes clear the need to focus future investigations away from proof-of-principle studies towards developing a better understanding of the roles that environmental factors and lipid composition and polymorphic phase behavior played in the origin and evolution of cell membranes.
Similar content being viewed by others
All life is chemistry
Jan Baptist van Helmont, Oratus medicinae, 1648
Introduction
The demise of the theory of spontaneous generation in mid-1800 triggered science’s search for an historical explanation for the origins of life. Since Stanley Miller’s 1952 production of biomonomers (Miller 1953), experiments designed to mimic the early Earth’s conditions have yielded many of life’s essential building blocks. The production of these building blocks, while crucial, is perhaps not the most critical step in the origin of life. According to Daniel Segré and Doron Lancet,
“It would thus be legitimate to dissociate the question of the origin of organics from that of the origin of life. The crucial enigma seems to be related to self-organization and the self-reproduction of supramolecular structures, and not to organosynthesis” (Segré and Lancet 2000).
To date, most origins-of-life research efforts have centered on the generation of self-replicating molecules and the emergence of proto-metabolic systems (Bada 2004; Orgel 1986; Lindahl 2002; Morowitz 1992; Walde 2006). By comparison, limited attention has been given to the self-organization of cellular boundaries (Walde 2006). Cellular boundaries or membranes are necessary for contemporary cells to function properly. Membranes act as a barrier to contain and concentrate the cell contents, selectively allow materials to flow into and out of the cell, and extract energy from the environment. Few would question if life, or at least life as we know it today, could exist without boundaries. Should the cell membrane be compromised, key processes of the cell are disrupted. Membrane formation is an essential step in the emergence of life.
Several researchers have recognized the importance of cell membrane formation to the origin-of-life process. For example, Morowitz and collaborators (Morowitz et al. 1988; Morowitz 1992) have suggested that primitive membranes had to emerge before self-replicating molecules and proto-metabolic systems and these vesicles constituted the first minimum protocell.
In a somewhat related vein, Lancet and co-workers (Segré et al. 2000, 2001) have proposed the lipid world scenario to explain the first steps in the origin of life. Like Morowitz and co-workers (Morowitz et al. 1988; Morowitz 1992), Lancet’s team has argued that aggregates comprised of amphiphiles served as the first protocell, emerging prior to self-replicating polymers and proto-metabolic networks.
While membrane structure has not been the main focus of origins-of-life research, pioneering work to explain the composition, structure, and function of the earliest membranes has been carried out by researchers since the 1950s. As Monnard and Deamer have noted (Monnard and Deamer 2002; Oparin 1924) was the first to propose that early life began as self-assembling organic material. This was followed in 1929 by Haldane’s (1929) conjecture that membranes played a key role in the origins of life. Actual laboratory studies on early membranes did not begin until the late 1950’s with the initial experiments involving aggregated colloidal particles (Oparin 1957). Goldacre (1958) explored the idea of membrane formation from lipid-like surfactants while Oparin continued studying heterogeneous spherical aggregates called coacervates (Oparin et al. 1976). The unlikelihood that coacervates were the precursor to modern cell membranes was soon discovered, however, due to their instability, lack of a permeability barrier, and the inability to encapsulate metabolism. It wasn’t until 1965 when Bangham and co-workers studied the spontaneous formation of bilayer vesicles from phospholipids that more contemporary ideas on the origins of cell membranes were inaugurated (Bangham et al. 1965). Currently researchers in laboratories such as those of Deamer et al. (2002), Szostak et al. (2001), Luisi et al. (2004), and Ourisson and Nakatani (1994), are spearheading the efforts to understand the emergence and evolution of cell membranes. Still, much remains to be done.
The purpose of this paper is to summarize and critically discuss the research to date on the prebiotic evolution of cell membranes and to highlight possible areas of future inquiry. We will show through a review of the literature that the pathway leading to the formation of primitive cell membranes and the transition to contemporary membranes appears to be sensitive to environmental effects and systematically and consistently depends on amphiphilic composition and phase behavior. Currently, little research focuses on understanding the role that these three factors played in the origin and evolution of cell membranes.
Cell Membranes
Typical contemporary cell membranes are formed from proteins dispersed in a matrix of phospholipids. Phospholipids can form abiotically when fatty acids, glycerin, and phosphate are moderately heated (65°C) to dryness. While complex phospholipids can be synthesized under hypothetical early-Earth conditions (Hargreaves et al. 1977; Hargreaves and Deamer 1978; Eichberg et al. 1977; Epps et al. 1978, 1979; Rao et al. 1982, 1987), Monnard and Deamer (2002) have pointed out that the simultaneous presence of fatty acids, glycerol, and phosphate on the early Earth is highly unlikely. The relevance of a reaction requiring complete dehydration under early-Earth conditions is also questionable. Deamer et al. (2002) have concluded that it is improbable that phospholipids were available on the prebiotic Earth. This indicates that the first cell boundaries were likely compositionally distinct from contemporary cell membranes or that membranes formed subsequent to the appearance of phospholipids.
Most origin of life researchers agree that the development of a cell boundary was an early step in the emergence of prebiotic cells. Regardless of the membrane composition, bilayer formation would be necessary to contain the cell contents and support transport and energy acquisition. Alkyl phosphates, alkyl sulfates, fatty acids, and amphiphilic hydrocarbons of at least eight carbons (Deamer 1998; Deamer et al. 1994), form bilayers under highly specific conditions. Apart from a role in membrane origins, these surfactants could provide the necessary environment for chemical reaction, compartmentalization, selective permeability, entrapment of catalysts or information rich molecules, and vesicle budding and fission (Walde 2006). Once these primitive membranes formed and became functional, the next step would be to transition into the phospholipid-based system characteristic of contemporary biological membranes.
Steps for Cell Membrane Origins and Evolution
Likely steps in the development of modern cell membranes include:
-
Production and/or delivery of prebiotic amphiphiles to early Earth
-
Self-assembly of the amphiphiles into vesicle systems
-
Encapsulation of self-replicating and/or protometabolic molecules/systems
-
Emergence of transport systems to allow materials into and out of the protocell
-
Generation of energy acquisition systems
-
Formation of mechanisms for membrane growth and protocell division
-
Transition from primitive membranes to contemporary membranes
Proof-of-principle experiments have demonstrated the physicochemical plausibility of these steps in cell membrane evolution. They also indicate that the processes involved in membrane origins are sensitive to the environment and influenced by lipid composition and phase behavior.
Prebiotic Sources of Amphiphiles
Proposed origins for membrane amphiphiles include both terrestrial and extraterrestrial sources (Bada 2004; Cronin 1998; Deamer 1985; Deamer and Pashley 1989). Amphiphiles have been recovered in meteorites and have been generated under a wide variety of conditions in the laboratory, ranging from simulated ultraviolet irradiation of interstellar ice particles to hydrothermal processing under simulated early-Earth conditions (Cronin 1998; Hanczyc et al. 2003).
The Fischer–Tropsch reaction has been known since the 1930s as a method to convert carbon monoxide and hydrogen into long-chain hydrocarbons in the presence of iron, cobalt or nickel at high temperatures. Workers have identified modifications to this process that yield amphiphilic compounds such as fatty acids and fatty alcohols (Zubay 2000) under aqueous conditions at relatively moderate temperatures (150–250°C) (Rushdi and Simoneit 2001). These conditions are potentially relevant to the early Earth, since they model the conditions at deep-sea hydrothermal vents, a possible site for prebiotic amphiphilic compound production. Rushdi and Simoneit (2006) have also shown that fatty acids will combine with ethylene glycol and glycerol under model hydrothermal conditions (150–250°C) to form ethylene glycolyl alkanoates and bis-alkanoates, and monoacylglycerols and diacylglycerols, respectively. As will be noted later, mono-acylglycerols may have played a role in prebiotic membrane evolution.
Hazen and Deamer (2006) have demonstrated that under hydrothermal vent temperatures and pressures pyruvic acid forms a complex mixture of organic materials. Some of these compounds are amphiphilic and can promote vesicle formation from nonpolar compounds found in the mixture. Currently it is not clear if these reactions are relevant to an early Earth setting with the authors acknowledging that further experiments are necessary to address whether these reactions could occur under plausible geothermal conditions.
Alternatively, Arthur Weber (1991) has proposed a synthetic cycle to account for prebiotic fatty acid production. This complex cycle starts with glycoaldehyde (a compound that likely existed on the early Earth) and proceeds through a series of steps that involve either loss of water or the addition of hydrogen.
Guy Ourisson and collaborators have developed a highly original proposal for the origin of primitive cell membranes. They suggest that instead of being comprised of alkyl chains, the first membrane lipids consisted of highly branched polyprenyl chains (Ourisson and Nakatani 1999). Presumably, primitive phosphate-based polyprenyl lipids could be generated from the acid-catalyzed condensation of isopentanol followed by phosphorylation. The isopentanol could be prebiotically produced via the acid catalyzed reaction between isobutene and formaldehyde (Ariga et al. 2005). It is questionable, however, if the simple materials for this prebiotic chemistry would have been at sufficient concentrations (Ourisson and Nakatani 1999) and if phosphorylating agents were available on early Earth (Keefe and Miller 1995).
The infall of extraterrestrial materials to the early Earth is also considered a source of bilayer-forming compounds. Deamer (1985) and Deamer and Pashley (1989) have shown that lipid-like materials extracted from the Murchison meteorite form bilayer structures under specific solution conditions. While lipid components may contain long-chain hydrocarbon moieties, analysis of carbon-containing meteorites (carbonaceous chondrites) indicated that not all of the meteorites examined contained long-chain hydrocarbons (Deamer et al. 1994) and it can’t be completely ruled out that the presence of long-chained hydrocarbons could be due to contamination (Cronin 1998). Laboratory experiments reveal that ultraviolet light irradiation of simulated cometary and interstellar ice (water, methanol, ammonia, and carbon monoxide) also produces a complex mixture of compounds that includes bilayer-forming materials (Dworkin et al. 2001).
While chemical synthesis and the possibility of extraterrestrial delivery of prebiotic amphiphiles have been demonstrated, it is not clear if these various pathways could yield the amphiphilic concentrations necessary for successful membrane formation. Deamer raises questions concerning the amount of organics present by infall and terrestrial synthesis and the kinetics of the turnover processes that balance the synthesis and degradation (by hydrolysis, photochemical degradation, and pyrolysis) of the accumulated organic material (Deamer 1997). This paper will assume that the materials to form protocellular membranes were sufficiently available on early Earth.
Self-assembly Into Vesicles
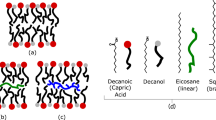
Simple amphiphiles, composed of a single hydrocarbon chain, will self-assemble into a variety of structures depending on concentration, composition, and environmental factors. While amphiphiles typically form spherical micelles, above a critical concentration (critical bilayer concentration [CBC]) prebiotically relevant compounds can form bilayer vesicles that are in equilibrium with single molecules and micelles. As Walde (2006) has pointed out, with the exception of cationic surfactants, vesicles generated from amphiphiles are metastable systems that do not form spontaneously with out mechanical or chemical input. Amphiphilic candidates other than fatty acids, i.e. polyprenyl derivatives (Nomura et al. 2001), have been proposed for prebiotic membranes but have been all but eliminated by the lack of plausible synthetic pathways (Monnard and Deamer 2002; Ourisson and Nakatani 1999). Research has shown that octanoic acid is the shortest monocarboxylic acid capable of forming a stable lipid bilayer (Apel et al. 2002). Carbon chains from 8 to 18 have been studied (Hargreaves and Deamer 1978; Apel et al. 2002), all resulting in bilayer formation when the pH and temperature are appropriately adjusted (Hargreaves and Deamer 1978). Shorter chain fatty acids form vesicles at room temperature, while chain lengths of 12 or larger require temperatures near the melting point of the acid (30–70°C) to form liposomes. The pH must be adjusted to within half a pH unit of the pKa of the acid, with a range of pHs of approximately 7 to 10 for carbons 8 to 18 (Hargreaves and Deamer 1978; Apel et al. 2002). For chain lengths longer than 12 carbons, the pH for vesicle formation is temperature-dependent (Hargreaves and Deamer 1978). Walde and co-workers (Namani and Walde 2005; Namini et al. 2007) have also noted the strict pH dependence of vesicle formation for decanoic acid and cis-4,7,10,13,16,19-docosahexaenoic acid (DHA). Decanoic/decanoate mixtures form vesicles when the pH is between 6.8 and 7.8. DHA forms vesicles only when the pH is kept between 8.5 and 9.2. It is unlikely that DHA has any relevance to the origin of life.
Luisi and co-workers (Blöchliger et al. 1998; Rasi et al. 2004) have also observed a pH dependence on vesicle formation for oleic acid. These investigators noted that the transformation from micelles to vesicles is influenced by the presence of pre-existing vesicles, a phenomenon termed the matrix effect. The kinetics of vesicle formation from a micelle system suggest that this process is autocatalytic, due to the matrix interactions between pre-existing micelles and surfactant molecules in solution.
The formation of stable bilayers is also affected by concentration with shorter-chain fatty acids needing a higher concentration for vesicles to form. In general, vesicles produced from fatty acids require relatively high concentrations, in the millimolar range (Deamer and Oró 1980). If the aqueous phase is diluted below critical bilayer concentrations (CBC), the vesicles are disrupted. CBC values range from approximately 130 to 20 mM for C8–C12 fatty acids.
Fatty acid bilayers can be stabilized over a larger alkaline pH range and lower concentration when fatty alcohols with the same hydrocarbon chain length as the fatty acid (Apel et al. 2002; Monnard et al. 2002) or when monoglycerides (Monnard and Deamer 2003) are added. Nonanoic acid only forms stable vesicles at pH 7.0 with a concentration >85 mM while nonanoic acid/nonanol mixture allows stable vesicle formation up to pH 11 at a concentration ≈20 mM. This effect, however, is only observed over a narrow range of molar ratios, ≈10/1 acid/alcohol (Monnard and Deamer 2003). For glycerol monooleate/decanoic acid mixtures, stable bilayers form at pH 7.4 for concentrations less than 67.5 mM total lipid, at ratios less than 3:1 fatty acid: glycerol monooleate (Monnard and Deamer 2003). Namani and Walde (2005) have noted a similar stabilization of decanoic acid vesicles when equimolar quantities of sodium dodecylbenzenesufonate (SDBS) are added. This mixed system forms vesicles below a pH of 6.8. Clearly, SDBS has little bearing on origin-of -life scenarios.
Initial work indicates that environmental factors also influence pure and mixed fatty acid bilayer stability (Hargreaves and Deamer 1978; Apel et al. 2002; Deamer and Oro 1980; Monnard and Deamer 2003). In addition to strict pH requirements, temperature effects, and relatively high concentrations, ionic strength and the presence of divalent cations in the aqueous medium inhibit bilayer formation (Monnard et al. 2002). If the ionic concentration, i.e., NaCl, increases above a threshold value, vesicles aggregate into sheets and the presence of divalent cations, Ca+2, Mg+2, Fe+2, thought to be present in the early oceans at 2 mM or higher, causes fatty acids and other amphiphiles to precipitate (Monnard and Deamer 2003). If the cell membrane forms, it must then be maintained in the fluid state. Many functions associated with contemporary membranes are hindered if the bilayer is in the gel state (Singer and Nicolson 1972). Consequently, the physical state of cell membranes is regulated through chain length, unsaturation, and chain branching. Presumably the primitive cell membrane must also meet this requirement (Deamer et al. 1994).
Similar environmental and composition effects have been noted by Ourisson and co-workers for vesicles formed from phosphorylated polyprenyl lipids (Ariga et al. 2005; Takajo et al. 2001; Gotoh et al. 2006). Lipids with at least three prenyl units form vesicles, but require highly exacting solution conditions that depend on the specific structural make-up of the polyprenyl moieties. A complex interrelationship exists between pH requirements for vesicle formation, monoanion/dianion ratio and the lengths of the hydrophobic chains and position of the double bonds. This interrelationship appears to be dictated by the hydrophilicity/ lipophilicity ratio of the phosphate headgroup to the hydrophobic chains.
Ariga et al. (2005) have suggested that phosphate based polyprenyl lipids could have formed the first primitive membrane vesicles from monolayers at the air/ water interface of the Earth’s oceans. Vesicle formation mediated by the collapse of these insoluble lipid monolayers, however, displays a dependence on cation composition and concentration in the subphase
While amphiphile composition greatly affects primitive membrane formation and stability (Monnard and Deamer 2003), the enhanced stability gained by addition of fatty alcohols or monoglycerides to fatty acid bilayer vesicles occurs only over a narrow compositional range and only for fatty alcohols that have hydrocarbon chains that closely match the chain length of the fatty acid. In addition, the presence of divalent cations negates the affect for the fatty alcohol addition (Monnard and Deamer 2002), suggesting that the presence of fatty alcohols on early Earth may not have had a large influence on primitive membrane formation.
Research indicates that primitive membranes would form only under highly constrained conditions on the early Earth and likely could not tolerate much in the way of environmental fluctuations. These results on nonphospholipid self-assembly provide promising initial insights into primitive membrane evolution. They also raise questions. How relevant, for example, are these studies to the events on early Earth that led to the origin of life? The production of pure and mixed fatty acid bilayer vesicles requires fairly fastidious procedures (Monnard and Deamer 2003). It is not clear how these procedures relate to conditions and processes on early Earth. No work to date has been done on the long-term stability of pure and mixed fatty acid vesicles. Single unilamellar vesicles made from phosphatidylcholine, for example, form a metastable phase that reverts to multilamellar sheets and vesicle phases in a short period of time (Lentz et al. 1987). Is this the case for fatty acid bilayer systems? Work by Walde (2006) seems to indicate that this is the case. To answer these and other questions raised by current research, more work is needed to sort out the relationship between the early Earth’s environment and primitive membrane formation as well as the effect of amphiphiles and other organic compounds on fatty acid bilayer formation and stability.
If nothing else, these pioneering studies indicate that the mere presence of amphiphiles on early Earth doesn’t automatically guarantee the emergence of primitive membranes; instead they point to a much more complex scenario for this critical step in life’s origin. This research also reveals how remarkably rich the phase behavior is for even simple amphiphiles. This phase behavior undoubtedly holds important clues to membrane formation and evolution.
Encapsulation/Transport
Once a stable bilayer is formed, relevant molecules must be encapsulated and some form of transport system, either active or passive, must be established. It is vital that the cell membrane enclose the materials necessary to sustain ‘life’ and future cell growth and division while allowing only specific chemicals to move into and out of the cell.
Most of the research has been done on encapsulation of molecules within phospholipid membranes (Deamer and Barchfield 1982; Chakrabarti and Deamer 1994; Chakrabarti et al. 1994; Paula et al. 1996), with relatively few studies involving encapsulation using fatty acid systems. This is clearly a deficiency given the proposed importance of fatty acids for the emergence of primitive cell membranes. Due to the structure of phospholipids, encapsulation requires a complex process involving detergent solubilization, sonication, and extrusion through polycarbonate filters. Phospholipid bilayer permeability to ions (potassium, phosphate and amino acids) has a half-life measured in days (Deamer 1997). The first prebiotic cells would need a more facile means of encapsulating macromolecules and greater permeability to necessary ions and nutrients.
A mechanism for encapsulation that could succeed in the early Earth environment for either phospholipids or fatty acid bilayers involves drying–wetting cycles where the concentrated amphiphiles fuse and sandwich the solute molecules during the drying cycle. Upon rewetting, the original solute is encapsulated into the amphiphilic vesicle, with the encapsulation efficiency ranging from 3–16% depending on the solute (Deamer and Barchfield 1982; Deamer et al. 1994; Shew and Deamer 1985; Apel and Deamer 2005). DNA is an exception with an encapsulation efficiency of approximately 50% for phospholipid systems (Deamer and Barchfield 1982). This type of drying–wetting cycle could have occurred at the intertidal zone or the edges of ponds or lakes. Decanoic acid vesicles have been studied using a similar method but in a simulated hydrothermal environment (Furuuchi et al. 2005). These vesicles could decompose and reform as the reaction medium flows past the hot and cold regions surrounding hydrothermal vents and in the process encapsulate monomers. Another possible prebiotic mechanism for encapsulation involves agitation of the vesicles, which break and reseal, capturing the solute particles. Encapsulation could similarly result if the vesicle is ruptured through an osmotic salt-concentration gradient and solute particles leak inward, being captured as the membrane is resealed when osmotic equilibrium is reestablished (Deamer et al. 1994).
Once information-rich genetic material and proto-metabolic systems are encapsulated, nutrients need a reliable pathway to get into and out of the protocell without repeatedly rupturing the membrane. The contemporary cell membrane’s phospholipid bilayer is a formidable barrier to most ionized substrates. Nutrients such as amino acids and phosphates are transported into the cell through protein-mediated processes. It is reasonable to assume that the first cells would lack the efficient membrane transport systems found in contemporary cell membranes (Deamer 1997).
The relative impermeability of phospholipid bilayers can be overcome by utilizing lipids with shorter chain hydrocarbons. Decreasing phospholipid chain length from 18 to 14 carbons increases the permeability to ions nearly 100 times due to defects that become common as the membrane thins (Paula et al. 1996; Deamer 1997). Added permeability in phospholipid systems is attained through impurities so that early membranes composed of mixed amphiphilic chains of 12–14 carbons would allow ionic nutrient solutes easy passage to take part in polymerization reactions while maintaining the encapsulated macromolecules (Deamer et al. 1994; Deamer 1997). These membranes must also protect the encapsulated substance. Chakrabarti et al. (1994) reveal that with judicious choice of lipid composition and environmental factors, permeability can be adjusted to support the activity of an encapsulated polymerase while protecting it from the environment, two necessary roles of cell membranes. Dimyristoyl phosphatidylcholine (DMPC) was used to encapsulate polynucleotide phosphorylase (PNase). ADP diffused across the DMPC membrane initiating the production of RNA by the PNase while protecting the enzyme from the externally added protease. Studies with decanoic acid/decanol vesicles also disclose that encapsulated catalase does not degrade in the presence of protease found outside the vesicle. Protease’s inability to diffuse across the membrane was demonstrated by the addition of catalase after vesicle formation. The external catalase was completely inhibited within 6 h while the encapsulated catalase was fully functional (Apel et al. 2002).
The encapsulation research involving phospholipid membranes (Deamer and Barchfield 1982; Deamer et al. 1994; Shew and Deamer 1985; Baeza et al. 1986) raises some interesting questions on the compositional dependence of this process. For encapsulation to occur, for example, strict concentrations and ratios of solute to lipid appear to be required. The theoretical limit for volume encapsulated by close-packed spheres is 74% (Shew and Deamer 1985). Experimental encapsulation efficiency of phospholipids ranged from 3 to 16% (DNA being a notable exception at around 50%) with the concentration of the solute in the 0.04 to 2 mg/ml range and the solute /lipid mass ratio from 1:250 to 1:2.5 (Shew and Deamer 1985). Shew and Deamer noted that dehydration/hydration depends on specific mass ratios of phospholipid to solute. These workers also demonstrated that this process is frustrated by the presence of inorganic ions indicating that environmental factors influence encapsulation. The process of repeated dehydration/hydration to encapsulate molecules may not be viable for phospholipids in a prebiotic environment containing a dilute solute in a ‘sea’ of ions. Any problem for a phospolipid system would be more acute for vulnerable fatty acid bilayers which readily lose integrity in response to changes in pH or temperature and the in presence of ions.
Environmental factors can also affect efficient encapsulation of molecules into fatty acid vesicles. Encapsulation by temperature flux around hydrothermal vents presents strict minimal temperature and ionic strength conditions. In order to maintain the vesicle, the temperature must be above the melting point of the acid (Furuuchi et al. 2005) and the concentration of bivalent cations and ionic strength must be low (Monnard et al. 2002). For decanoic acid vesicles the temperature must be above 32°C in the cold regions surrounding the vents. Furuuchi et al. theorize that unsaturated fatty acids, with lower melting points, may have taken over the membrane structure as the temperature surrounding the vents decreased, which led to an eventual conversion to a phospholipid membrane that could more easily tolerate cold environments. The problem with this, they acknowledge, is that from a prebiotic standpoint it is unknown how (and unlikely that) unsaturated fatty acids and phospholipids could be synthesized (Furuuchi et al. 2005).
A final concern for encapsulation involves the loss of vesicles and encapsulated material upon subsequent dehydration/hydration or cold/hot cycles. To date, the focus has been on the initial encapsulation event. What happens upon subsequent dehydration/hydration cycles? Is the original encapsulated material lost? If so, what percentage loss occurs? Does the system reach steady state? These are critical questions that need to be addressed.
The emergence of a transport system to service encapsulated molecules was necessary for the developing protocell. Passive transport or diffusion is a simple method for exchange of building blocks, nutrients, and waste where no metabolic energy is required.
Permeability studies with phospholipid bilayers indicate that amphiphilic composition plays a key role in transport across the membrane (Paula et al. 1996; Chakrabarti and Deamer 1992; Deamer 1997; Vlassov 2005; Khvorova et al. 1999; Vlassov et al. 2001; Janas et al. 2004). The chain-length dependence on membrane permeability has already been noted. Research involving transport systems using proposed fatty acid membranes is sparse. Experiments by Sacerdote and Szostak (2005) and by Hargreaves and Deamer (1978) also suggest the importance of bilayer composition for fatty acid systems, although only unsaturated fatty acids, materials not likely found on early Earth, were used in their studies. A subsequent experiment, again suggesting a compositional dependence, by Apel et al. (2002) used a decanoic acid/decanol membrane to test enzyme activity. It appears that vesicles involving saturated 12–14 carbon fatty acids are ideal for formation and encapsulation and are likely components of prebiotic membranes (Deamer 1997). In contrast to this requirement, the experiments conducted to ascertain if monomers/nutrients are easily encapsulated into vesicles relied on unsaturated acids or phospholipids.
Passive transport forces an inherent difficulty in protocell development. If equilibrium is reached, no further exchange of materials can occur (Trevors 2003). Unless there is continuous change in the external environment, the protocell would in effect ‘die.’ While a prebiotic soup-like environment would provide the necessary variation in material and concentrations, it could work negatively by removing needed substances in the environment, causing the cell to lose access to vital nutrients. This further limits the location/environment of biogenesis by requiring an exacting but ever-changing concentration of the correct substances necessary for the appearance of the first cells ready for growth and replication.
Energy Acquisition
Cell membranes play a key role in harvesting energy for cellular use. Sources for prebiotic energy acquisition could include heat energy, chemical energy, and light energy (Deamer 1997). Heat energy could be used to drive condensation reactions; chemical energy could take the form of chemical condensing agents, pyrophosphate bond energy, synthesis of glyceraldehydes (Weber 1987), and pyrite catalyzed reactions; light energy, the most abundant source of energy on prebiotic Earth, could be captured through reactions involving iron compounds or polycyclic aromatic hydrocarbons (PAHs).
While all these methods are viable sources of energy, none seem appropriate for a cellular system bounded by a fatty acid membrane with known prebiotic chemicals present in the environment (Deamer 1997).
Modern cells employ proton gradients to harvest energy necessary to sustain life by a chemiosmotic process. While pure fatty acids will self-assemble, encapsulate macromolecules, and allow passive transport, their carboxyl head group mediates proton permeability, eliminating the possibility of a proton gradient (Chen and Szostak 2004a). A small amount of a fatty acid incorporated into a phospholipids bilayer will disrupt the pH gradient within seconds (Chen and Szostak 2004a; Gutknecht 1988; Pohl et al. 2000; Zhang et al. 1996). One study has been carried out using unsaturated fatty acid vesicles in which an ion gradient was generated through membrane growth. Maintenance of a pH gradient necessitated an oleate-arginine system, arginine being used to slow the decay of the gradient. In addition to this compositional restriction, the use of unsaturated hydrocarbons (which were not likely present on early Earth) raises questions about the prebiotic relevance of this experiment. The oleate-arginine system also requires surroundings substantially free of alkali cations, again an unlikely scenario in light of the probable environment on the early Earth.
Light, while the most abundant and most easily accessible source of energy on the surface of the early Earth, requires a light-sensitive chemical to absorb and then convert the light energy into a form of energy useful to the prebiotic cell. This assumes some type of pigment system was available to the cell (Deamer et al. 1994). Iron compounds have been shown to cause a shift in pH of up to 3 units when irradiated with near-UV light (Deamer 1997; Deamer and Harang 1990). If the iron compounds are encapsulated, this change produces a pH gradient that could be converted to usable energy. Another proposed pigment system involves polycyclic aromatic hydrocarbons (PAH), which also absorb light in the near-UV and blue region. In the 1992 study by Deamer, pyrene and fluoranthene, PAHs found in the Murchison meteorite, were mixed with hexadecane and then illuminated. The production of amphiphilic molecules indicates that PAHs could have served as a primitive pigment system capturing light energy to drive the reaction. During this process a proton is released, supporting the possibility of PAHs generating an electrochemical gradient across a membrane. Several models have been proposed to trap the released proton in the form of a chemiosmotic gradient (Deamer 1997). While substantial amounts of iron compounds were presumably present on the early Earth and PAHs have been found to make up 90% of the kerogen-like organic material of carbonaceous chondrites (Deamer 1997; Cronin et al. 1988), studies to date have been conducted with phospholipid bilayers (Deamer 1997, 1992; Deamer and Harang 1990), not fatty acid vesicles. Because these reactions involve a proton gradient, they do not seem to be relevant to energy acquisition by cells bounded by fatty acid membranes due to the proton permeability of these systems.
Because of the inability of saturated fatty acid vesicles to sustain a proton gradient, either another as-yet unknown form of energy harvesting was present in the prebiotic cell or
“the ability to use energy stored in pH gradients may not have been possible until the evolution of membranes composed of less permeable membrane components, such as phosphate or glycerol esters, and with relatively low steady-state levels of free fatty acids” (Chen and Szostak 2004a).
Growth/Division
Once primitive cell membranes formed, they had to grow and spontaneously divide without the aid of biomolecular machinery (Hanczyc et al. 2003).
According to a proposal by Morowitz (Morowitz et al. 1988; Morowitz 1992), once the membraneous vesicle emerged, additional amphiphilic components could be synthesized within the membrane from materials in the environment leading to the growth and subsequent division into smaller vesicles after the original parent vesicle reached an unstable size. In this proposal the energy to drive the continuous synthesis of amphiphilic components would have come from hydrophobic chemophores that partitioned into the vesicle membrane and mediated energy harvesting photochemical processes.
Early research on self-replicating micelles (Bachmann et al. 1991, 1992) and vesicles (Walde et al. 1994; Morigaki et al. 1997) supports the mechanism suggested by Morowitz. Membrane precursors (e.g., water-insoluble anhydride of the fatty acid) are hydrolyzed within the boundary of already formed vesicles yielding the same fatty acid surfactant. The additional surfactants form new vesicles when the critical concentration is reached. The process is autocatalytic, with the rate of formation of vesicles increasing as the concentration of the vesicles increases. Because this process does not involve ‘budding’ vesicles (although the existence of ‘budding’ vesicles was shown), the relationship to self-reproduction in protocellular systems is probably limited. (Walde et al. 1994)
Hanczyc and co-workers report several methods for vesicle growth. By the slow addition, to prevent new vesicle formation, of one equivalent of myristoleate micelles to a myristoleic acid/ myristoleate vesicle system, 90% of the added fatty acid was incorporated into the original vesicles (Hanczyc and Szostak 2004). Another pathway involves the exchange of single chain amphiphiles between vesicles due to a difference in osmotic pressure. Isotonic vesicles can lose membrane material to osmotically swollen oleate vesicles resulting in a 35% loss/gain in surface area (Chen et al. 2004). Growth by vesicle to vesicle fusion would have an advantage by bringing together two sets of encapsulated materials and new membrane components but while much work has been done on the fusion of phospholipid vesicles, the fusion of fatty acid vesicles has not been explored.
These results demonstrate that in principle vesicle growth is possible through physicochemical processes. The specific fatty acids studied are not probable on early Earth, raising questions as to the geochemical relevance of these mechanisms. Another possible problem arises when the mechanisms for fatty acid growth and division are considered in the context of the RNA world. Ribozymes require the presence of divalent cations which can cause fatty acids to precipitate. Chen et al. (2005) has shown that myristoleic acid and the glycerol monoester of the acid form vesicles that are tolerant to Mg+2. The trade off is that leads to a reduced yield of vesicle growth following micelle addition. This again highlights the compositional dependence of the origin and evolution of cell membranes.
Fission, vesicle self-reproduction related to cell processes, occurs when the vesicle is transformed into a budded shape of two spheres connected by a narrow neck that splits into two daughter vesicles (Božič and Svetina 2004). In order for this to occur, the vesicle must grow by incorporating new material into its membrane. Chen and Szostak have studied the growth of fatty acid vesicles through the addition of fatty acid micelles. The results correlate the ratio of micelle/vesicle to the kinetics of cell growth. It was found that for a low micelle/vesicle ratio, <0.4, growth depends on the micelle concentration and occurs in a single phase, presumably the incorporation of the micelles into the preformed vesicles. If the micelle/vesicle ratio exceeded 0.4, growth occurred in two phases with the fast first phase depending on the vesicle concentration. The preformed vesicles experienced ≈40% increase in surface area regardless of the micelle concentrations. In the second phase, growth of the vesicles proceeded more slowly with a presumed competition for the micelles forming de novo vesicles. This phase is dependent on micelle concentration suggesting an autocatalytic reaction, the micelles aggregating to form vesicles (Chen and Szostak 2004b; Walde et al. 1994; Berclaz et al. 2001). Because this mechanism involves micelles being incorporated into the bilayer through electrostatic and entropic affects, temperature and ionic strength could presumably affect the reaction (Chen and Szostak 2004b).
Once a vesicle has experienced growth and reaches a certain size, fission may occur. According to Božič and Svetina (2004), the ability to attain the budded vesicle shape, to self-reproduce, is related to the time it takes to double the membrane’s surface area and other physical properties of the vesicles components (the spontaneous curvature, membrane hydraulic permeability, etc.). From this, a mathematical relationship based on a simple spontaneous curvature model can be developed where only those vesicles which meet the criteria will increase their numbers by self-reproduction. Self-reproduction can occur only for selected values of the membrane material constants, so it is inherently related to the composition of the membrane.
Although this research is insightful, it fails to establish a connection between vesicle replication in the laboratory and vesicle replication under conditions of the prebiotic Earth (Maddox 1994). These studies on growth and reproduction involve unsaturated fatty acid membranes. Since it is unlikely that these compounds were present on the early Earth, it raises further questions about the prebiotic relevance of these experiments. Still, this research provides the physicochemical proof of principle for self-reproduction of fatty acid vesicles. It also points to the importance of membrane composition and the influence of environmental factors.
Modern Membranes
At some point in the prebiotic evolution of cell membranes, phospholipid-based membranes must emerge from primitive systems. It is reasonable to assume that the first phospholipid species on the early Earth, and hence the first contemporary cell membranes consisted of phosphatidylethanolamines (PEs) and phosphatidylglycerols (PGs). PEs and PGs stand as the dominant phospholipid species in bacterial membranes, the first life-forms to appear on Earth (Rottem 1982), so origin-of-life scenarios must specifically account for the formation of biological membranes composed of these two phospholipid types. While these phospholipids self-assemble into bilayers, they also form non-bilayer structures as well. Phospholipids tend to form specific aggregate types based on the area of the headgroup relative to the volume of the hydrocarbon moieties (Israelachvili et al. 1980). PEs tend to form non-bilayer phases, and in the presence of Ca2+, PGs also form non-bilayer structures (Seddon 1990; Lindblom and Rilfors 1989). Cardiolipin, a derivative of PGs also present in bacterial membranes, likewise readily forms non-bilayer states. Finally, PGs presence in PE aggregates increases PEs tendency to form non-bilayer aggregates (Rana 1990). These non-bilayer phases compromise the cell membrane’s structural integrity and, with it, its barrier function (Seddon 1990). Permanent or long-lived non-bilayer phases would quickly lead to loss of cell integrity. For example, work on Acholeplasma laidlawii demonstrated that if these bacteria did not alter their lipid composition as environmental conditions changed, the cell membrane would adopt non-bilayer structures which would lead to cell death (Wieslander et al. 1980).
The presence of Ca2+ in the early Earth’s environment would cause PEs and PE/PG mixtures to form non-bilayer aggregates. This tendency to form non-bilayer phases would have frustrated the pathway leading to the first contemporary cell membranes. Moreover, early bilayers composed of PEs and PGs would have held the potential to transition to non-bilayer phases in response to fluctuating environmental conditions and altered bilayer composition.
Even if the first phospholipids on Earth were the ones that exclusively favored bilayer phases, spontaneous assembly of cell membrane systems would not necessarily be automatic. Bilayer-forming phospholipids display complex polymorphic phase behavior aggregating into a wide range of bilayer structures that include multilamellar sheets and vesicles (Lasic 1988). These multilamellar bilayer phases are not true representations of contemporary cell membranes which consist exclusively of unilamellar bilayer phases. Laboratory manipulation (Lasic 1988) is required to form unilamellar vesicles for most phospholipid species. Unilamellar vesicles consisting of phosphatidylcholine, for example, exist only for a limited time as a metastable phase eventually fusing into multilamellar sheets or vesicles (Lentz et al. 1987). Formation of stable, unilamellar bilayer phases, like those of contemporary cell membranes, is a critical phenomenon. For pure phospholipid systems, multilamellar bilayer phases spontaneously transform into stable unilamellar phases at a critical temperature that depends on the identity of the phospholipid for pure systems and on phospholipid composition for mixed systems (Gershfeld 1989a, b; Gershfeld et al. 1993).
Support for the critical nature of single-bilayer membranes comes from a series of studies. Ginsberg et al. (1991) noted that phospholipids extracted from rat and squid nervous-system tissue assemble into a stable unilamellar phase at critical temperatures that correspond to the physiological temperatures of these two organisms. For cold-blooded sea urchins, L. pictus, to maintain a single bilayer phase in embryonic cells, membrane composition varies such that the critical temperature matches environmental conditions (Tremper and Gershfeld 1999). E. coli also must adjust its cell membrane phospholipid composition to maintain the unilamellar phase (Jin et al. 1999). Deviation from critical conditions triggers disruption of the single bilayer, causing cell death. Other studies indicate the harmful effects that occur when cell membranes deviate from critical conditions. Gershfeld noted a correlation between the rupture of human red blood cells and incubation at temperatures exceeding 37°C. Transformation of the cell membrane from a single bilayer to multiple bilayer stacks accompanies the rupture of the red blood cells (Gershfeld and Murayama 1988).
Cell membranes seem to be exacting molecular structures that depend on a precise set of physical and chemical conditions (Gershfeld 1989a, b; Gershfeld et al. 1993; Ginsberg et al. 1991; Tremper and Gershfeld 1999; Jin et al. 1999; Gershfeld and Murayama 1988). Studies on the critical nature of contemporary cell membranes raise questions about the likelihood of chemical and physical processes operating on the early Earth producing the necessary phospholipid composition to form the stable single bilayer phase that universally defines cell membranes. Even if physicochemical processes produced the exact phospholipid composition, any fluctuations in temperature would destroy the single bilayer structure. So, although phospholipids readily aggregate in many cases to form bilayer structures, only very specific phospholipid compositions and exacting environmental temperatures appear to lead to unilamellar bilayer structures and cell membranes.
Conclusion
One of the most important stages in the origin and early development of life was the formation of cellular membranes. In spite of its critical significance, researchers have paid relatively little attention to this aspect of the origin-of-life problem. Only a handful of pioneering research teams has made an effort at understanding the emergence of cell membranes. Their work has primarily centered on proof-of-principle experiments designed to assess the feasibility of the different steps in membrane origins and evolution.
Further, the amount of experimental data decreases as the research focus proceeds through the proposed stages in the origin of cell membranes from the source of prebiotic amphiphiles to self assembly through growth/division and energy acquisition. The majority of the studies have used pure amphiphiles, not the mixtures that would likely be found on prebiotic Earth. In the case of energy acquisition and growth/division, the research to date primarily involves phospholipids or unsaturated fatty acid vesicles, substances not expected to be present on the prebiotic Earth. We are not aware of any research that has used saturated fatty acids, the most probable composition of early prebiotic membranes, for experiments involving energy transduction or growth/division of prebiotic cells.
The objective of this study was to critically assess the research to date on cell membrane origins with the goal of highlighting a possible direction for future inquiry. In doing so, we have discovered that virtually every step in the process of membrane origins and evolution appears to be crucially influenced by environmental conditions, and lipid composition and polymorphic phase behavior. While researchers have noted the influence of these factors on the emergence of cell membranes, their pervasiveness has largely gone unrecognized.
The environment dictates whether an amphiphile will form bilayers. For single-chain, simple amphiphiles, phase behavior related to bilayer formation is affected by a narrow range of conditions involving temperature, pH, divalent cation concentration, and ionic strength. The most accepted method for encapsulation involves repeated dehydration/hydration cycles in tide pools or small ponds. This process also concentrates any ions present, disrupting the bilayer reformation. In addition, for passive transport to occur, the pH and temperature must be adjusted according to the specific amphiphile present in the bilayers and there must be a constant supply of the necessary nutrients/building blocks. The mechanism for vesicle growth involves electrostatic and entropic affects dependent upon temperature and ionic strength while the phase behavior for PEs or PGs, likely the first phospholipids available, is highly affected by divalent cation concentration and temperature.
Compositional constraints influence formation of stable bilayers made up of single chain amphiphiles. The stability of the bilayer can be enhanced by the addition of other compounds but only for very specific amphiphile/compound mole ratios. In order for passive transport to occur across the bilayer, fatty acid chain length is restricted to between 12 and 14 carbons. Encapsulation requires a specific solute to lipid ratio, and vesicle growth is affected by the vesicle/micelle ratio. Prebiotic cell membranes, thought to be composed of saturated fatty acids, could not hold a proton gradient, so energy acquisition would have been delayed until more complicated amphiphiles were present. Cell division studies on unsaturated fatty acid vesicles relate specific amphiphile physical constants with the ability to undergo fission.
While precise membrane composition and environmental factors may be regulated in individual steps, major difficulties arise when trying to integrate the conditions essential for each step into a cohesive stepwise evolution. It is almost like having 50 pieces to a puzzle and finding no two pieces fit together because they are from 50 different puzzles. Likewise, the conditions necessary for each step of the transition from free saturated fatty acids to modern cell membranes does not “fit” together to form the completed puzzle. Some of the problem areas include:
-
A concentrating mechanism would be needed to reach the CBC of the amphiphile, yet most concentrating methods would also concentrate any ionic solute present, inhibiting self-assembly.
-
The requirement of low ion concentration for bilayer formation necessitates fresh water, as the ionic strength of the early oceans is speculated to be greater than that in modern oceans. There was likely little or no fresh water on the prebiotic Earth (Monnard et al. 2002).
-
In order to encapsulate molecules, the dehydration/hydration process would need to be carried out in tidal areas or small ponds. While enclosing necessary molecules, this process would also concentrate any ions present, disrupting the bilayer.
-
Energy acquisition creates a major difficulty in membrane origins. Saturated fatty acid vesicles will not hold a proton gradient, believed to be the most likely form of early energy capture. Unsaturated fatty acids, while able to hold a proton gradient, are not likely compounds present on early Earth. Phospholipids, modern membrane components, probably arose only after metabolism. This creates the problem of needing phospholipids first to hold the proton gradient in metabolism but needing metabolism first to produce phospholipids.
Proof-of-principle experiments indicate that physicochemical processes could conceivably lead to the origin and birth of cell membranes, but environmental and lipid compositional fluctuations on early Earth could hinder the emergence of cell membrane systems and the transition to contemporary cell membranes. Future investigations need to concentrate on developing a better understanding of the role that environmental conditions, and lipid composition and phase structure play in membrane origins.
References
Apel CL, Deamer DW (2005) The formation of glycerol monodecanoate by a dehydration/condensation reaction: increasing the chemical complexity of amphiphiles on the early Earth. Orig Life Evol Biosph 35:323–332
Apel CL, Deamer DW, Mautner MN (2002) Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim Biophys Acta 1559:1–9
Ariga K, Yuki H, Kikuchi J, Dannemuller O, Albrecht-Gary A, Nakatani Y, Ourisson G (2005) Monolayer studies of single-chain polyprenyl phosphates. Langmuir 21:4578–4583
Bachmann PA, Walde P, Luisi PL, Lang J (1991) Self-replicating micelles:aqueous micelles and enzymatically driven reactions in reverse micelles. J Am Chem Soc 113:8204–8209
Bachmann PA, Luisi PL, Lang J (1992) Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 357:57–59
Bada JL (2004) How life began on Earth: a status report. Earth Planet Sci Letters 226:1–15
Baeza I, Ibañez M, Santiago JC, Wong C, Lazcano A, Oró J (1986) Studies on precellular evolution: the encapsulation of polyribonucleotides by liposomes. Adv Space Res 6(11):39–43
Bangham AD, Standish MM, Miller N (1965) Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13:238–252
Berclaz N, Müller M, Walde P, Luisi PL (2001) Growth and transformation of vesicles studied by ferritin labeling and cryotransmission electron microscopy. J Phys Chem 105:1056–1064
Blöchliger E, Blocher M, Walde P, Luisi PL (1998) Matrix effect in the size distribution of fatty acid vesicles. J Phys Chem 102:10383–10390
Božic B, Svetina S (2004) A relationship between membrane properties forms the basis of a selectivity mechanism for vesicle self-reproduction. Eur Biophys J 33:565–571
Chakrabarti AC, Deamer DW (1992) Permeability of lipid bilayers to amino acids and phosphate. Biochim Biophys Acta 111:171–177
Chakrabarti AC, Deamer DW (1994) Permeation of membranes by the neutral form of amino acids and peptides: relevance to the origin of peptide translocation. J Mol Evol 39:1–5
Chakrabarti AC, Breaker RR, Joyce GF, Deamer DW (1994) Production of RNA by a polymerase protein encapsulated within phospholipid vesicles. J Mol Evol 39:555–559
Chen IA, Szostak JW (2004a) Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc Natl Acad Sci 101:7965–7970
Chen IA, Szostak JW (2004b) A kinetic study of the growth of fatty acid vesicles. Biophys J 87:988–998
Chen IA, Roberts RW, Szostak JW (2004) The emergence of competition between model protocells. Science 305:1474–1476
Chen IA, Salehi-Ashtiani K, Szostak JW (2005) RNA catalysis in model protocell vesicles. J Am Chem Soc 127:13213–13219
Cronin JR (1998) Clues from the origin of the solar system: meteorites. In: Andre B (ed) The molecular origin of life: assembling pieces of the puzzle. Cambridge University Press, Cambridge, UK, pp 119–146
Cronin JR, Pizzarello S, Cruickshank DP (1988) Organic matter in carbonaceous chondrites, planetary satellites, asteroids and comets. In: Kerridge JF, Matthews MS (eds) Meteorites and the early solar system. University of Arizona Press, Tucson, pp 819–857
Deamer DW (1985) Boundary structures are formed by organic compounds of the Murchinson carbonaceous chondrites. Nature 317:792–794
Deamer DW (1992) Polycyclic aromatic hydrocarbons: primitive pigment systems in the prebiotic environment. Adv Space Res 12(4):183–189
Deamer DW (1997) The first living systems: a bioenergetic perspective. Microbiol Mol Biol Rev 61(2):239–261 (June)
Deamer DW (1998) Membrane compartments in prebiotic evolution. In: Andre B (ed) The molecular origins of life: assembling the pieces of the puzzle. Cambridge University Press, Cambridge, UK, pp 189–205
Deamer DW, Barchfield GL (1982) Encapsulation of macromolecules by lipid vesicles under simulated prebiotic conditions. J Mol Evol 18:203–206
Deamer DW, Harang E (1990) Light-dependent pH gradients are generated in liposomes containing ferrocyanide. Biosystems 24:1–4
Deamer DW, Oró J (1980) Role of lipids in prebiotic structures. BioSystems 12:167–175
Deamer DW, Pashley RM (1989) Amphiphilic components of the Murchinson carbonaceous chondrite: surface properties and membrane formation. Orig Life Evol Biosph 19:21–38
Deamer DW, Mahon EH, Bosco G (1994) Self-assembling and function of primitive membrane structures. In: Bengtson S (ed) Early life on Earth, Nobel Symposium, No. 84. Columbia University Press, New York, pp 107–115
Deamer DD, Jason P, Sandford SA, Berstein MP, Allamandola LJ (2002) The first cell membranes. Astrobiology 2(4):375
Dworkin JP, Deamer DW, Sandford SA, Allamandola LJ (2001) Self-assembling amphiphilic molecules: synthesis in simulated interstellar/precometary ices. Proc Natl Acad Sci 98(3):815–819
Eichberg J, Sherwood E, Epps DE, Oró J (1977) Cyanamide mediated syntheses under plausible primitive Earth conditions. IV. The synthesis of acylglycerols. J Mol Evol 10:221–230
Epps DE, Sherwood E, Eichberg J, Oró J (1978) Cyanamide mediated syntheses under plausible primitive Earth conditions. V. The synthesis of phosphatadic acid. J Mol Evol 11:279–292
Epps DE, Nooner DW, Eichberg J, Sherwood E, Oró J (1979) Cyanamide mediated syntheses under plausible primitive Earth conditions. VI. The synthesis of glycerol and glycerolphosphates. J Mol Evol 14:235–241
Furuuchi R, Imai E-I, Honda H, Hatori K, Matsuno K (2005) Evolving lipid vesicles in prebiotic hydrothermal environments. Orig Life Evol Biosph 35:333–343
Gershfeld NL (1989a) The critical unilamellar lipid state: a perspective for membrane bilayer assembly. Biochim Biophys Acta 988:335–350
Gershfeld NL (1989b) Spontaneous assembly of a phospholipid bilayer as a critical phenomenon: influence of temperature, composition, and physical state. J Phys Chem 93:5256–5264
Gershfeld NL, Murayama M (1988) Thermal instability of red blood cell membrane bilayers: temperature dependence of hemolysis. J Mem Biol 101:67–72
Gershfeld NL, Mudd CP, Tajima K, Berger RL (1993) Critical temperature for unilamellar vesicle formation in dimyristoyl phosphatidylcholine dispersions from specific heat measurements. Biophys J 65:1174–1179
Ginsberg L, Gilbert DL, Gershfeld NL (1991) Membrane bilayer assembly in neural tissue of rat and squid as a critical phenomena: influence of temperature and membrane proteins. J Mem Biol 119:65–73
Goldacre RJ (1958) Surface films: their collapse on compression, the shapes and sizes of cells, and the origin of life. In: Danielli JF, Pankhurst KGA, Riddiford AC (eds) Surface phenomena in biology and chemistry. Pergamon, New York, pp 12–27
Gotoh M, Mike A, Nagano H, Ribeiro N, Elhabiri M, Gumienna-Kontecka E, Albrecht-Gary A, Schmutz M, Ourisson G, Nakatani Y (2006) Mambrane properties of branched polyprenyl phosphates, postulated as primitive membrane constituents. Chem Bio Diversity 3:434–455
Gutknecht J (1988) Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J Mem Biol 106:83–93
Haldane JBS (1929) The origin of life. The Rationalist Annual 148:3–10
Hanczyc MM, Szostak JW (2004) Replicating vesicles as models of primitive cell growth and division. Curr Opin Chem Biol 8:660–664
Hanczyc MM, Fujikawa SM, Szostak JW (2003) Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science 302:618
Hargreaves WR, Deamer DW (1978) Liposomes from ionic, single-chain amphiphiles. Biochemistry 17:3759–3768
Hargreaves WR, Mulvihill S, Deamer DW (1977) Synthesis of phospholipids and membranes in prebiotic conditions. Nature 266:78–80
Hazen RM, Deamer DW (2006) Hydrothermal reactions of pyruvic acid:synthesis, selection, and self-assembly of amphiphilic molecules. Orig Life Evol Biosph [Epub ahead of print]
Israelachvili JN, Marčelja S, Horn RG (1980) Physical principles of membrane organization. Q Rev Biophys 13:121–200
Janas T, Janas T, Yarus M (2004) A membrane transporter for tryptophan composed of RNA. RNA 10:1541–1549
Jin AJ, Edidin M, Nossal R, Gershfeld NL (1999) A singular state of membrane lipids at cell growth temperatures. Biochemistry 38:13275–13278
Keefe AD, Miller SL (1995) Are polyphoshates or phosphate esters prebiotic reagents? J Molec Evol 41:693–702
Khvorova A, Kwak Y-G, Tamkun M, Majerfeld I, Yarus M (1999) RNA’s that bind and change the permeability of phospolipid membranes. Proc Natl Acad Sci U S A 96:10649–10654
Lasic DD (1988) The mechanism of vesicle formation. Biochemistry J 256:1–11
Lentz BR, Carpenter TJ, Alford DR (1987) Spontaneous fusion of posphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry 26:5389–5397
Lindahl PA (2002) Stepwise evolution of nonliving to living chemical systems. Orig Life Evol Biosph 34:371–389
Lindblom G, Rilfors L (1989) Cubic phases and isotropic structures formed by membrane lipids-possible biological relevance. Biochim Biophys Acta 988:221–256
Luisi PL, Stano P, Rasi S, Mavelli F (2004) A possible route to prebiotic vesicle reproduction. Artif Life 10:297–308
Maddox J (1994) Origin of the first membrane? Nature 371:101
Martin W, Russell MJ (2003) On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil Trans R Soc Lond B 358:62–63
Miller SL (1953) Production of amino acids under possible primitive Earth conditions. Science 117:528–529
Monnard P-A, Deamer DW (2002) Membrane self-assembly processes: steps toward the first cellular life. Anat Rec 268:197
Monnard P-A, Deamer DW (2003) Preparation of vesicles from nonphospholipid amphiphiles. Methods Enzymol 372:133–151
Monnard P-A, Apel CL, Kanavarioti A, Deamer DW (2002) Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: implications for a prebiotic aqueous medium. Astrobiology 2:139–152
Morigaki K, Dallavalle S, Walde P, Colonna S, Luisi PL (1997) Autopoietic self-reproduction of chiral fatty acid vesicles. J Am Chem Soc 119:292–301
Morowitz HJ (1992) The beginnings of cellular life: metabolism recapitulates biogenesis. Yale University Press, New Haven, CT
Morowitz HJ, Heinz B, Deamer DW (1988) The chemical logic of a minimum protocell. Orig Life Evol Biosph 18:281–287
Namani T, Walde P (2005) From decanoate micelles to decanoic acid/dodecylbenzenesulfonate vesicles. Langmuir 21:6210–6219
Namini T, Ishikawa T, Morigaki K, Walde P (2007) Vesicles from docosahexaenoic acid. Colloids Surf 54:118–123
Nomura SM, Yoshikawa Y, Yoshikawa K, Dannenmuller O, Chasserot-Golaz S, Ourisson G, Nakatani Y (2001) Towards proto-cells: “primitive” lipid vesicles encapsulating giant DNA and its histone complex. ChemBiochem 6:457–459
Oparin AI (1924) The origin of life. Moscow: Izd. Moskovshii Robochii. English translation: Vernal JD (1967). The origin of life. Weidenfeld and Nicolson, London, pp 199–234
Oparin, AI (1957) The origin of life on earth. Academic, New York
Oparin, AI, Orlovskii AF, Bukhlaeve VY, Gladilin KL (1976) Influence of the enzymatic synthesis of polyadenylic acid on a coacervate system. Dokl Akad Nauk SSSR 226:972–974
Orgel LE (1986) RNA catalysis and the origins of life. J Theor Biol 123:127–149
Ourisson G, Nakatani T (1994) The terpenoid theory of the origin of cellular life: the evolution of terpenoids to cholesterol. Chem Biol 1:11–23
Ourisson G, Nakatani Y (1999) Origins of cellular life: molecular foundations and new approaches. Tetrahedron 55:3183–3190
Paula S, Volkov G, Van Hoek AN, Haines TH, Deamer DW (1996) Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys J 70:339–348
Pohl EE, Peterson U, Sun J, Pohl P (2000) Changes of intrinsic membrane potentials induced by flip-flop of long-chain fatty acids. Biochemistry 39:1834–1839
Rana FR (1990) Structure and function of outer membranes and LPS from Wild-Type and LPS-Mutant strains of Salmonella typhimurium and their interaction with Magainins and Polymyxin B. Dissertation, Ohio University
Rao M, Eichberg J, Oró, J (1982) Synthesis of phosphatidylcholine under possible primitive Earth conditions. J Mol Evol 18:196–202
Rao M, Eichberg J, Oró J (1987) Synthesis of phosphatidyethanolamine under possible primitive Earth conditions. J Mol Evol 25:1–6
Rasi S, Mavelli F, Luisi PL (2004) Matrix effect in oleate micelles-vesicles transformation. Orig Life Evol Biosph 34:214–224
Rottem S (1982) Transbilayer distribution of lipids in microbial membranes. In: Rezin S, Ralter R (eds) Current topics in membranes and transport 17. Academic, New York, pp 235–261
Rushdi AI, Simoneit BRT (2001) Lipid formation by aqueous Fischer–Tropsch type synthesis over a temperature range of 100 to 400°C. Orig Life Evol Biosph 31:103–118
Rushdi AI, Simoneit BRT (2006) Abiotic condensation synthesis of glyceride lipids and wax esters under simulated hydrothermal conditions. Orig Life Evol Biosph 36:93–108
Sacerdote MG, Szostak JW (2005) Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc Natl Acad Sci 102(17):6004–6008
Seddon JM (1990) Structure of the inverted hexagonal (H11) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta 1031:1–69
Segré D, Lancet D (2000) Composing Life. EMBO Rep 11:218
Segré D, Ben-Eli D, Lancet D (2000) Compositional genomes: prebiotic information transfer in mutually catalytic non-covalent assemblies. Proc Natl Acad Sci U S A 97(8):4112–4117
Segré D, Ben-Eli D, Deamer D, Lancet D (2001) The lipid world. Orig Life Evol Biosph 31:119–145
Shew RL, Deamer DW (1985) A novel method for encapsulation of macromolecules in liposomes. Biochim Biophys Acta 816:1–8
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Szostak JW, Bartel DP, Luisi PL (2001) Synthesizing life. Nature 409:387–390
Takajo S, Nagano H, Dannenmuller O, Ghosh S, Albrecht AM, Nakatanni Y, Ourisson, G (2001) Membrane properties of sodium 2- and 6-(poly)prenyl-substituted polyprenyl phosphates. New J Chem 25:917–929
Tremper KE, Gershfeld NL (1999) Temperature dependence of membrane lipid composition in early blastula embryos of Lutechinus pictus: selective sorting of phospholipids into nascent plasma membranes. J Membrane Biol 171:47–53
Trevors JT (2003) Possible origin of a membrane in the subsurface of the Earth. Cell Biol Int 27:451–457
Vlassov A (2005) How was membrane permeability produced in an RNA world? Orig Life Evol Biosph 35:135–149
Vlassov A, Khvorova A, Yarus M (2001) Binding and disruption of phospholipids bilayers by supramolecular RNA complexes. Proc Natl Acad Sci 98(14):7706–7711
Walde P (2006) Surfactant assemblies and their various possible roles for the origin(s) of life. Orig Life Evol Biosph 36:109–150
Walde P, Wick R, Fresta M, Mangone A, Luisi PL (1994) Autopoietic self-reproduction of fatty acid vesicles. J Am Chem Soc 116:11649–11654
Weber AL (1987) The triose model: glyceraldehyde as a source of energy and monomers for prebiotic condensation reactions. Orig Life 17:107–119
Weber AL (1991) Origin of fatty acid synthesis: thermodynamics and kinetics of reaction pathways. J Mol Evol 32:93–100
Wieslander A, Christiansson A, Rilfors L, Lindblom, G (1980) Lipid bilayer stability in membranes, regulation of lipid composition in Acholeplasma laidlawii as governed by molecular shape. Biochemistry 19:3650–3655
Zhang F, Kamp F, Hamilton JA (1996) Dissociation of long and very long chain fatty acids from phospholipid bilayers. Biochemistry 35:16055–16060
Zubay G (2000) Orgins of Life on the Earth and in the Cosmos, 2nd edn. Academic, San Diego, CA, p 347
Acknowledgements
We wish to acknowledge the Southwestern College Sabbatical Committee and School Board, and RTB for their financial support for JT and FRR, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, J.A., Rana, F.R. The Influence of Environmental Conditions, Lipid Composition, and Phase Behavior on the Origin of Cell Membranes. Orig Life Evol Biosph 37, 267–285 (2007). https://doi.org/10.1007/s11084-007-9065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-007-9065-6