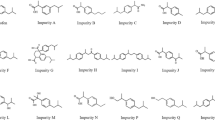
The purpose of this work was to develop a sensitive and validated HPLC method for analysis of rivaroxaban and estimation of related impurities in parent drug substance and pharmaceutical dosage forms. The analysis was carried out on a Macherey-Nagel Nucleodur C18 column (250 × 4.6 mm, 5 μm particle size) with a mobile phase containing acetonitrile and water in gradient program at a flow rate of 1.5 mL/min, the column oven temperature 55°C, and the UV detector wavelength set at 254 nm. The method was validated according to USP38-NF33 guideline recommendations and to the ICH guidelines for validation. The linearity, selectivity, accuracy, precision, robustness, LOD and LOQ characteristics of the proposed method showed acceptable values. The method is suitable for practical routine analysis of drug substance and pharmaceutical dosage forms, and it was successfully used to analyze Rovaltro (Syrian product) and Xarelto (brand product). All the analysis results were acceptable according to pharmaceutical requirements.






Similar content being viewed by others
References
Xarelto (Rivaroxaban) Summary of Product Characteristics, Bayer Pharma AG (2013).
Xarelto Summary of Product Characteristics, Bayer Pharma AG (2015).
S. Görög, Identification and Determination of Impurities in Drugs, Elsevier (2000).
P. Rao, V. Cholleti, and V. Reddy, Int. J. Res. Pharm. Sci., 5, 17 – 24 (2015).
P. Satyanarayana and A. S. Madhavi, Int. J. Sci. Innov. Discov., 2, 226 – 231 (2012).
M. Çelebier, T. Reçber, E. Koçak, and S. Altinöz, Braz. J. Pharm. Sci., 49, 359 – 366 (2013).
K. Chandra, P. Satya, A. Dhana, et al., Res Desk, 1, 24 (2012).
D. Vaghela, P. Patel, D. Vaghela, and P. Patel, Int. J. Pharm. Pharm. Sci., 6, 383 – 386 (2014).
C.B. Sekaran, V. H. Bind, M. R. Damayanthi, and A. Sireesha, Pharma Chemica, 5, 1 – 5 (2013).
G. Rohde, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 872, 43 – 50 (2008).
United States Pharmacopeia-National Formulary, USP 38-NF 33, Rand McNally, Rockville, USA (2015).
British Pharmacopoeia, Vol. V, XVII G, H, and XII B1, British Pharmacopoeia Commission, London (2013), A487.
European Pharmacopoeia, 7th Edition (2010).
I. H. T. Guideline, Validation of Analytical Procedures: Text and Methodology, Q2 (R1), 1 (2005).
Xarelto: Summary of Product Characteristics, Bayer Schering Pharma AG (2008).
I. H. T. Guideline, Impurities in New Drug Substances, Q3A (R2), in: Proceedings of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland (2006).
Acknowledgments
The authors are thankful to colleagues in the central laboratory of the Faculty of Science for their cooperation in carrying out this work. The authors would like to thank Dr. Heba Ghazal for her help in funding the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arous, B., Al-Mardini, M.A., Karabet, F. et al. Development and Validation of a Liquid Chromatography Method for the Analysis of Rivaroxaban and Determination of Its Production Related Impurities. Pharm Chem J 52, 483–490 (2018). https://doi.org/10.1007/s11094-018-1844-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1844-z