Abstract
Purpose
The objective of this study was to characterize efflux proteins (P-glycoprotein (P-gp), multidrug resistance proteins (MRP1–6) and breast cancer resistance protein (BCRP)) of retinal pigment epithelium (RPE) cell lines.
Methods
Expression of efflux proteins in two secondary (ARPE-19, D407) and two primary (HRPEpiC and bovine) RPE cell lines was measured by quantitative RT-PCR and western blotting. Furthermore, activity of MRP1 and MRP5 of ARPE-19 cell line was assessed with calcein-AM and carboxydichlorofluorescein (CDCF) probes.
Results
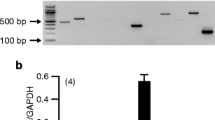
Similar efflux protein profile was shared between ARPE-19 and primary RPE cells, whereas D407 cell line was notably different. D407 cells expressed MRP2 and BCRP, which were absent in other cell lines and furthermore higher MRP3 transcript expression was found. MRP1, MRP4 and MRP5 were identified from all human RPE cell lines and MRP6 was not expressed in any cell lines. The pattern of efflux protein expression did not change when ARPE-19 cells were differentiated on filters. The calcein-AM and CDCF efflux tests provided evidence supporting MRP1 and MRP5 activity in ARPE-19 cells.
Conclusions
MRP1, MRP4 and MRP5 are the main efflux transporters in RPE cell lines. There are differences in efflux protein expression between RPE cell lines.






Similar content being viewed by others
References
Steuer H, Jaworski A, Elger B, Kaussmann M, Keldenich J, Schneider H, et al. Functional characterization and comparison of the outer blood–retina barrier and the blood–brain barrier. Invest Ophthalmol Vis Sci 2005;46:1047–53. doi:10.1167/iovs.04-0925.
Cunha-Vaz JG. The blood–retinal barriers system. Basic concepts and clinical evaluation. Exp Eye Res 2004;78:715–21. doi:10.1016/S0014-4835(03)00213-6.
Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood–retina barrier: Emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev 2006;58:1136–63. doi:10.1016/j.addr.2006.07.024.
Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv 2004;1:99–114. doi:10.1517/17425247.1.1.99.
Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci 2005;46:641–6. doi:10.1167/iovs.04-1051.
Maurice DM, Mishima S. Ocular Pharmacokinetics.Berlin: Springer; 1984. p. 19–116.
Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 2003;55:3–29. doi:10.1016/S0169-409X(02)00169-2.
Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976;455:152–62. doi:10.1016/0005-2736(76)90160-7.
Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD. Transport of bile acids, sulfated steroids, estradiol 17-beta-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol Pharmacol 2005;67:545–57. doi:10.1124/mol.104.007138.
Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol 2003;63:1094–103. doi:10.1124/mol.63.5.1094.
Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol 2006;46:381–410. doi:10.1146/annurev.pharmtox.46.120604.141238.
Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem 2002;71:537–92. doi:10.1146/annurev.biochem.71.102301.093055.
Esser P, Tervooren D, Heimann K, Kociok N, Bartz-Schmidt KU, Walter P, et al. Intravitreal daunomycin induces multidrug resistance in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1998;39:164–70.
Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vis 2002;8:422–30.
Constable PA, Lawrenson JG, Dolman DE, Arden GB, Abbott NJ. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp Eye Res 2006;83:24–30.
Aukunuru JV, Sunkara G, Bandi N, Thoreson WB, Kompella UB. Expression of multidrug resistance-associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a novel aldose reductase inhibitor. Pharm Res 2001;18:565–72. doi:10.1023/A:1011060705599.
Cai H, Del Priore LV. Bruch membrane aging alters the gene expression profile of human retinal pigment epithelium. Curr Eye Res 2006;31:181–9. doi:10.1080/02713680500514628.
Stojic J, Stohr H, Weber BH. Three novel ABCC5 splice variants in human retina and their role as regulators of ABCC5 gene expression. BMC Mol Biol 2007;8:42. doi:10.1186/1471-2199-8-42.
Geisen P, McColm JR, King BM, Hartnett ME. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Curr Eye Res 2006;31:739–48. doi:10.1080/02713680600837408.
Luo Y, Zhuo Y, Fukuhara M, Rizzolo LJ. Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Invest Ophthalmol Vis Sci 2006;47:3644–55. doi:10.1167/iovs.06-0166.
Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 1996;62:155–69. doi:10.1006/exer.1996.0020.
Dunn KC, Marmorstein AD, Bonilha VL, Rodriguez-Boulan E, Giordano F, Hjelmeland LM. Use of the ARPE-19 cell line as a model of RPE polarity: basolateral secretion of FGF5. Invest Ophthalmol Vis Sci 1998;39:2744–49.
Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, Jeanny JC, et al. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis 2005;11:124–32.
Guillonneau X, Tassin J, Berrou E, Bryckaert M, Courtois Y, Mascarelli F. In vitro changes in plasma membrane heparan sulfate proteoglycans and in perlecan expression participate in the regulation of fibroblast growth factor 2 mitogenic activity. J Cell Physiol 1996;166:170–87. doi:10.1002/(SICI)1097-4652(199601)166:1<170::AID-JCP19>3.0.CO;2-J.
Korjamo T, Honkakoski P, Toppinen MR, Niva S, Reinisalo M, Palmgren JJ, et al. Absorption properties and P-glycoprotein activity of modified Caco-2 cell lines. Eur J Pharm Sci 2005;26:266–79. doi:10.1016/j.ejps.2005.06.004.
Chen G, Duran GE, Steger KA, Lacayo NJ, Jaffrezou JP, Dumontet C, et al. Multidrug-resistant human sarcoma cells with a mutant P-glycoprotein, altered phenotype, and resistance to cyclosporins. J Biol Chem 1997;272:5974–82. doi:10.1074/jbc.272.9.5974.
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992;258:1650–54. doi:10.1126/science.1360704.
Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 1997;57:3537–47.
Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci USA 1999;96:6914–19. doi:10.1073/pnas.96.12.6914.
Adachi M, Sampath J, Lan LB, Sun D, Hargrove P, Flatley R, et al. Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J Biol Chem 2002;277:38998–9004. doi:10.1074/jbc.M203262200.
McAleer MA, Breen MA, White NL, Matthews N. pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem 1999;274:23541–548. doi:10.1074/jbc.274.33.23541.
Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J Biol Chem 2002;277:16860–867. doi:10.1074/jbc.M110918200.
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 1998;95:15665–670. doi:10.1073/pnas.95.26.15665.
Essodaigui M, Broxterman HJ, Garnier-Suillerot A. Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug resistance protein and P-glycoprotein. Biochemistry 1998;37:2243–50. doi:10.1021/bi9718043.
Pratt S, Chen V, Perry WI 3rd, Starling JJ, Dantzig AH. Kinetic validation of the use of carboxydichlorofluorescein as a drug surrogate for MRP5-mediated transport. Eur J Pharm Sci 2006;27:524–32. doi:10.1016/j.ejps.2005.09.012.
Tian X, Zamek-Gliszczynski MJ, Zhang P, Brouwer KL. Modulation of multidrug resistance-associated protein 2 (Mrp2) and Mrp3 expression and function with small interfering RNA in sandwich-cultured rat hepatocytes. Mol Pharmacol 2004;66:1004–10. doi:10.1124/mol.66.4.
Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turncliff RZ, Pollack GM, Brouwer KL. Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Ther 2003;304:801–9. doi:10.1124/jpet.102.044107.
Yu XQ, Xue CC, Wang G, Zhou SF. Multidrug resistance associated proteins as determining factors of pharmacokinetics and pharmacodynamics of drugs. Curr Drug Metab 2007l;8:787–802. doi:10.2174/138920007782798171.
Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Kock K, Kroemer HK. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev 2005;37:253–78. doi:10.1081/DMR-200047984.
Arshavsky VY, Lamb TD, Pugh EN Jr. G proteins and phototransduction. Annu Rev Physiol 2002;64:153–87. doi:10.1146/annurev.physiol.64.082701.102229.
Marmor MF, Negi A. Pharmacologic modification of subretinal fluid absorption in the rabbit eye. Arch Ophthalmol 1986;104:1674–77.
Kogishi JI, Akimoto M, Mandai M, Kuriyama S, Hall MO, Honda Y, et al. Nitric oxide as a second messenger in phagocytosis by cultured retinal pigment epithelial cells. Ophthalmic Res 2000;32:138–42. doi:10.1159/000055604.
Kuriyama S, Hall MO, Abrams TA, Mittag TW. Isoproterenol inhibits rod outer segment phagocytosis by both cAMP-dependent and independent pathways. Invest Ophthalmol Vis Sci 1995;36:730–6.
Norris MD, Smith J, Tanabe K, Tobin P, Flemming C, Scheffer GL, et al. Expression of multidrug transporter MRP4/ABCC4 is a marker of poor prognosis in neuroblastoma and confers resistance to irinotecan in vitro. Mol Cancer Ther 2005;4:547–53. doi:10.1158/1535-7163.MCT-04-0161.
Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci USA 2000;97:7476–81. doi:10.1073/pnas.120159197.
Cunha-Vaz JG, Maurice DM. The active transport of fluorescein by the retinal vessels and the retina. J Physiol 1967;191:467–86.
Barza M, Kane A, Baum J. Pharmacokinetics of intravitreal carbenicillin, cefazolin, and gentamicin in rhesus monkeys. Invest Ophthalmol Vis Sci 1983;24:1602–606.
Hosoya K, Tomi M. Advances in the cell biology of transport via the inner blood–retinal barrier: establishment of the cell lines and transport functions. Biol Pharm Bull 2005;28:1–8. doi:10.1248/bpb.28.1.
Greenwood J. Characterization of a rat endothelial cell culture and the expression of P-glycoprotein in brain and retinal endothelium in vitro. J Neuroimmunol 1992;39:123–32. doi:10.1016/0165-5728(92)90181-J.
Shen J, Cross ST, Tang-Liu DD, Welty DF. Evaluation of an immortalized retinal endothelial cell line as an in vitro model for drug transport studies across the blood–retinal barrier. Pharm Res 2003;20:1357–63. doi:10.1023/A:1025789606885.
Asashima T, Hori S, Ohtsuki S, Tachikawa M, Watanabe M, Mukai C, et al. ATP-binding cassette transporter G2 mediates the efflux of phototoxins on the luminal membrane of retinal capillary endothelial cells. Pharm Res 2006;23:1235–42. doi:10.1007/s11095-006-0067-2.
Tachikawa M, Toki H, Tomi M, Hosoya K. Gene expression profiles of ATP-binding cassette transporter A and C subfamilies in mouse retinal vascular endothelial cells. Microvasc Res 2008;75:68–72. doi:10.1016/j.mvr.2007.05.002.
Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 2000;25:228–31. doi:10.1038/76109.
Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest 2002;82:515–8.
Acknowledgements
We are grateful to Dr P. Borst (Netherlands Cancer Institute) for providing MDCKII and HEK293-MRP4 cell lines, Dr J. Mönkkönen (University of Kuopio) for his support in the completion of this work and Dr M. Suhonen (University of Kuopio) for providing compounds for calcein-AM studies. M.Sc. Sanna Siissalo is acknowledged for her help with the CDCFDA/CDCF-assay and M.Sc. Mika Reinisalo for his advices regarding qRT-PCR standards. We also thank Dr J. Taipalensuu and Lucia Lazorova (Uppsala University, Sweden) for providing the plasmids for quantitative RT-PCR measurement and original authors for the permissions to use plasmids: Dr. Branimir Sikic (Stanford University School of Medicine, USA), Dr. Susan Cole (Cancer Research Institute, Queen’s University, Kingston, Canada), Dr. Piet Borst (Netherlands Cancer Institute, The Netherlands), Dr. John Schuetz (St. Jude Children’s Research Hospital, Memphis, USA), Astellas Pharma Inc. (former Yamanouchi Pharmaceutical Co., Ltd), Dr. Andras Varadi and Dr. Attila Iliás (Hungarian Academy of Sciences, Hungary), Dr. Douglas Ross (University of Maryland, Marlene and Stewart Greenebaum Cancer Center, Baltimore, USA).
This work was supported by the Finnish Eye Foundation (EM, KK), the Finnish Eye and Tissue Bank Foundation (EM), the Evald and Hilda Nissi’s Foundation (EM), Sokeain Ystävät ry/De Blindas Vänner rf (EM), the Finnish Cultural Foundation of Northern Savo (EM), the Finnish Funding Agency for Technology and Innovation (TEKES) (KK, AU).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mannermaa, E., Vellonen, KS., Ryhänen, T. et al. Efflux Protein Expression in Human Retinal Pigment Epithelium Cell Lines. Pharm Res 26, 1785–1791 (2009). https://doi.org/10.1007/s11095-009-9890-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9890-6