ABSTRACT
Purpose
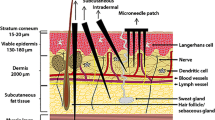
The aim of the study was to develop a cheap and fast method to produce hollow microneedles and an applicator for injecting vaccines into the skin at a pre-defined depth and test the applicability of the system for dermal polio vaccination.
Methods
Hollow microneedles were produced by hydrofluoric acid etching of fused silica capillaries. An electromagnetic applicator was developed to control the insertion speed (1–3 m/s), depth (0–1,000 μm), and angle (10°–90°). Hollow microneedles with an inner diameter of 20 μm were evaluated in ex vivo human skin and subsequently used to immunize rats with inactivated poliovirus vaccine (IPV) by an intradermal microinjection of 9 μL at a depth of 300 μm and an insertion speed of 1 m/s. Rat sera were tested for IPV-specific IgG and virus-neutralizing antibodies.
Results
Microneedles produced from fused silica capillaries were successfully inserted into the skin to a chosen depth, without clogging or breakage of the needles. Intradermal microinjection of IPV induced immune responses comparable to those elicited by conventional intramuscular immunization.
Conclusions
We successfully developed a hollow microneedle technology for dermal vaccination that enables fundamental research on factors, such as insertion depth and volume, and insertion angle, on the immune response.








Similar content being viewed by others
Abbreviations
- IPV:
-
Inactivated polio vaccine
- OPV:
-
Oral polio vaccine
- VN:
-
Virus neutralizing
REFERENCES
Bal SM et al. Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J Control Release. 2010;148(3):266–82.
Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–55.
Prausnitz MR,Gill HS, Park J-H. Microneedles for drug delivery. In: Modified release, drug delivery. 2008. p. 295–309.
Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–7.
Kew OM et al. Vaccine-derived poliovirusus and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59(1):587–635.
Salk JE, et al. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. 2011.
Fine PEM, Carneiro IAM. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol. 1999;150(10):1001–21.
Modlin JF. Poliomyelitis in the United States: the final chapter? JAMA: J Am Med Assoc. 2004;292(14):1749–51.
Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol. 2010;172(11):1213–29.
Nelson KS et al. Intradermal fractional dose inactivated polio vaccine: a review of the literature. Vaccine. 2012;30:121–5.
Nicolas J-F, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7(8):1201–14.
Chandrasekaran S, Frazier AB. Mechanical characterization of surface micromachined hollow metallic microneedles. IEEE The Sixteenth Annual International Conference on Micro Electro Mechanical Systems, 2003. MEMS-03 Kyoto, 2002: p. 363–366.
Davis SP et al. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52:909–15.
Gardeniers HJGE et al. Silicon micromachined hollow microneedles for Transdermal liquid transport. J Microelectromech Syst. 2003;12(3):855–62.
McAllister DV et al. Microfabricated needles for Transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100(24):13755–60.
Verbaan FJ et al. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J Control Release. 2008;128:80–8.
Westdijk J et al. Characterization and standardization of Sabin based inactivated polio vaccine: proposal for a new antigen unit for inactivated polio vaccines. Vaccine. 2011;29:3390–7.
van Steenis G, van Wezel A, Sekhuis V. Potency testing of killed polio vaccine in rats. Dev Biol Stand. 1981;47:119–28.
Westdijk J et al. Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains. Vaccine. 2013;31:1298–304.
Cormier M et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–11.
Wang PM et al. Precise microinjection into skin using hollow microneedles. J Investig Dermatol. 2006;126(5):1080–7.
Haq MI et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11(1):35–47.
Yang M, Zahn JD. Microneedle insertion force reduction using vibratory actuation. Biomed Microdevices. 2004;6(3):177–82.
Crichton ML et al. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials. 2010;31:4562–72.
Martanto W et al. Microinfusion using hollow microneedles. Pharm Res. 2006;23(1):104–13.
Roxhed N et al. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans Biomed Eng. 2008;55(3):1063–71.
Paik S-J et al. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sensors Actuators A Phys. 2004;2004:276–84.
Cui Q, Liu C, Zha XF. Study on a piezoelectric micropump for the controlled drug delivery system. Microfluid Nanofluid. 2007;3:377–90.
ACKNOWLEDGMENTS AND DISCLOSURES
Koen van der Maaden and Sebastiaan J. Trietsch contributed equally. We thank the Electronics Department at Leiden University for their help in the development of the microneedle applicator. Furthermore, we thank Aat Mulder for preparing the cryosections of rat skin and Pim Schipper for performing the microinjections into ex vivo human skin and the subsequent cryosections. This work was (co)financed by the Netherlands Metabolomics Centre (NMC), which is a part of The Netherlands Genomics Initiative/Netherlands Organization for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
(DOCX 44 kb)
Rights and permissions
About this article
Cite this article
van der Maaden, K., Trietsch, S.J., Kraan, H. et al. Novel Hollow Microneedle Technology for Depth-Controlled Microinjection-Mediated Dermal Vaccination: A Study with Polio Vaccine in Rats. Pharm Res 31, 1846–1854 (2014). https://doi.org/10.1007/s11095-013-1288-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1288-9