Abstract
Purpose
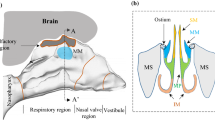
Aerosol particle deposition in the human nasal cavity is of high interest in particular for intranasal central nervous system (CNS) drug delivery via the olfactory cleft. The objective of this study was the development and comparison of a numerical and experimental model to investigate various parameters for olfactory particle deposition within the complex anatomical nasal geometry.
Methods
Based on a standardized nasal cavity, a computational fluid and particle dynamics (CFPD) model was developed that enables the variation and optimization of different parameters, which were validated by in vitro experiments using a constructed rapid-prototyped human nose model.
Results
For various flow rates (5 to 40 l/min) and particle sizes (1 to 10 μm), the airflow velocities, the calculated particle airflow patterns and the particle deposition correlated very well with the experiment. Particle deposition was investigated numerically by varying particle sizes at constant flow rate and vice versa assuming the particle size distribution of the used nebulizer.
Conclusions
The developed CFPD model could be directly translated to the in vitro results. Hence, it can be applied for parameter screening and will contribute to the improvement of aerosol particle deposition at the olfactory cleft for CNS drug delivery in particular for biopharmaceuticals.











Similar content being viewed by others
Abbreviations
- BBB:
-
Blood–brain barrier
- CAD:
-
Computer-aided design
- CFPD:
-
Computational fluid and particle dynamics
- CNS:
-
Central nervous system
- CT:
-
Computed tomography
- DNS:
-
Direct Numerical Simulations
- IP:
-
Impaction parameter
- LES:
-
Large Eddy Simulations
- MMAD:
-
Mass median aerodynamic diameter
- NALT:
-
Nasopharynx-associated lymphoid tissue
- RANS:
-
Reynolds-averaged Navier–Stokes
- WHO:
-
World Health Organization
References
World Health Organization and Alzheimer’s Disease International. Dementia: a public health priority. 2012.
World Health Organization. Neurological disorders: public health challenges. 2006.
Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104(1):29–45.
Craft S, Baker L, Montine T. Intranasal insulin therapy for Alzheimer Disease and amnestic mild cognitive impairment. 2012;69(1):29–38.
Mathison S, Nagilla R, Kompella UB. Nasal route for direct delivery of solutes to the central nervous system: fact or fiction? J Drug Target. 1998;5(6):415–41.
Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. Elsevier B.V.; 2012;64(7):614–28.
Stützle M, Flamm J, Carle S, Schindowski K. Nose-to-Brain delivery of insulin for Alzheimer’s disease. Admet Dmpk. 2015;not yet pu(3):190–202.
Holton N, Yokley T, Butaric L. The morphological interaction between the nasal cavity and maxillary sinuses in living humans. Anat Rec. 2013;296(3):414–26.
Holton NE, Yokley TR, Figueroa A. Nasal septal and craniofacial form in European- and African-derived populations. J Anat. 2012;221(3):263–74.
Ito T, Nishimura TD, Hamada Y, Takai M. Contribution of the maxillary sinus to the modularity and variability of nasal cavity shape in Japanese macaques. Primates. 2014;56(1):11–9.
Springer IN, Zernial O, Nölke F, Warnke PH, Wiltfang J, Russo PAJ, et al. Gender and nasal shape: measures for rhinoplasty. Plast Reconstr Surg. 2008;121(2):629–37.
Leong SCL, White PS. A comparison of aesthetic proportions between the Oriental and Caucasian nose. Clin Otolaryngol Allied Sci. 2004;29(6):672–6.
López B, Toro V, Schilling A, Suazo Galdames I. Nasal profile assessment using geometric morphometrics in a sample of Chilean population: clinical and forensic implications. Int J Morphol. 2012;30(1):302–8.
Zhu JH, Lee HP, Lim KM, Lee SJ, Wang DY. Evaluation and comparison of nasal airway flow patterns among three subjects from Caucasian, Chinese and Indian ethnic groups using computational fluid dynamics simulation. Respir Physiol Neurobiol. 2011;175(1):62–9.
Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1–2):1–24.
Vyas TK, Shahiwala A, Marathe S, Misra A. Intranasal drug delivery for brain targeting. Curr Drug Deliv. 2005;2(2):165–75.
Kiyono H, Fukuyama S. Nalt- versus peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4(9):699–710.
Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338(20):1405–12.
Hölscher C. First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer’s disease. Alzheimers Dement. 2014;10(1):33–7.
Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30(14):4999–5007.
Craft S, Claxton A, Baker L, Cholerton B, Hanson A, Callaghan M, et al. Therapeutic effects of long-acting intranasal insulin detemir for Alzheimer’s dementia or mild cognitive impairment. Alzheimers Dement. 2013;9(4):139–40.
Cheng KH, Cheng YS, Yeh HC, Guilmette RA, Simpson SQ, Yang YH, et al. In vivo measurements of nasal airway dimensions and ultrafine aerosol deposition in the human nasal and oral airways. J Aerosol Sci. 1996;27(5):785–801.
Salem H, Katz SA. Inhalation toxicology. Third Edit. Taylor and Francis; 2014. 623 p.
Richter G. Forced inspiratory nasal flow-volume curves: a simple test of nasal airflow. Mayo Clin Proc. 2001;76(10):990–4.
Kelly JT, Prasad AK, Wexler AS. Detailed flow patterns in the nasal cavity. J Appl Physiol. 2000;89(1):323–37.
Liu Y, Matida EA, Gu J, Johnson MR. Numerical simulation of aerosol deposition in a 3-D human nasal cavity using RANS, RANS/EIM, and LES. J Aerosol Sci. 2007;38(7):683–700.
Schroeter JD, Kimbell JS, Asgharian B, Tewksbury EW, Singal M. Computational fluid dynamics simulations of submicrometer and micrometer particle deposition in the nasal passages of a Sprague–Dawley rat. J Aerosol Sci. 2012;43(1):31–44.
Lindemann J, Brambs HJ, Keck T, Wiesmiller KM, Rettinger G, Pless D. Numerical simulation of intranasal airflow after radical sinus surgery. Am J Otolaryngol - Head Neck Med Surg. 2005;26(3):175–80.
Hertel SP, Winter G, Friess W. Protein stability in pulmonary drug delivery via nebulization. Adv Drug Deliv Rev. 2015;93:79–94.
Albu S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des Dev Ther. 2012;6:125–32.
Inthavong K, Fung MC, Yang W, Tu J. Measurements of droplet size distribution and analysis of nasal spray atomization from different actuation pressure. J Aerosol Med Pulm Drug Deliv. 2015;28(1):59–67.
Mori E, Merkonidis C, Cuevas M, Gudziol V, Matsuwaki Y, Hummel T. The administration of nasal drops in the “Kaiteki” position allows for delivery of the drug to the olfactory cleft: a pilot study in healthy subjects. Eur Arch Oto-Rhino-Laryngology. 2015;1–5.
Benchetrit G. Breathing pattern in humans: diversity and individuality. Respir Physiol. 2000;122(2–3):123–9.
Hudgel DW, Devadatta P, Hamilton H. Pattern of breathing and upper airway mechanics during wakefulness and sleep in healthy elderly humans. J Appl Physiol. 1993;74(5):2198–204.
Liu Y, Johnson MR, Matida EA, Kherani S, Marsan J. Creation of a standardized geometry of the human nasal cavity. J Appl Physiol. 2009;106(3):784–95.
Kundoor V, Dalby RN. Assessment of nasal spray deposition pattern in a silicone human nose model using a color-based method. Pharm Res. 2010;27(1):30–6.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5.
Menter FR. Two-equation eddy-viscosity turbulence models for engineering applications. AIAA J. 1994;32(8):1598–605.
Zubair M, Shuaib IL, Abdullah MZ, Hamid SA. Review : a critical overview of limitations of CFD modeling in nasal airflow. 2011;32(2):77–84.
Rosin P, Rammler E. The laws governing the fineness of powdered coal. J Inst Fuel. 1933;7(31):29–36.
Liu Y. Numerical and experimental analyses of aerosol deposition in a novel and standardized human nasal cavity. ottawa; 2010.
Lintermann A, Meinke M, Schröder W. Fluid mechanics based classification of the respiratory efficiency of several nasal cavities. Comput Biol Med. 2013;43(11):1833–52.
Schröder W. Human computational fluid dynamics: from the nose model to the real nose. Jahrbuch 2013 der Braunschweigischen Wissenschaftlichen Gesellschaft. 2014. p. 160–86.
Inthavong K, Tian ZF, Tu JY, Yang W, Xue C. Optimising nasal spray parameters for efficient drug delivery using computational fluid dynamics. Comput Biol Med. 2008;38(6):713–26.
Achilles N, Pasch N, Lintermann A, Schröder W, Mösges R. Computational fluid dynamics: a suitable assessment tool for demonstrating the antiobstructive effect of drugs in the therapy of allergic rhinitis. Acta Otorhinolaryngol Ital. 2013;33(1):36–42.
Churchill SE, Shackelford LL, Georgi JN, Black MT. Morphological variation and airflow dynamics in the human nose. Am J Hum Biol. 2004;16(6):625–38.
Zhao K, Jiang J. What is normal nasal airflow? A computational study of 22 healthy adults. Int Forum Allergy Rhinol. 2014;4(6):435–46.
Lee CF, Abdullah MZ, Ahmad KA, Lutfi Shuaib I. Standardization of Malaysian adult female nasal cavity. Comput Math Methods Med. 2013;2013:11.
Tan J, Han D, Wang J, Liu T, Wang T, Zang H, et al. Numerical simulation of normal nasal cavity airflow in Chinese adult: a computational flow dynamics model. Eur Arch Otorhinolaryngol. 2012;269(3):881–9.
Tu J, Inthavong K, Ahmadi G. Computational fluid and particle dynamics in the human respiratory system. 2013. 3883 p.
Keyhani K, Scherer PW, Mozell MM. Numerical simulation of airflow in the human nasal cavity. J Biomech Eng. 1995;117(4):429.
Inthavong K, Ge Q, Se CMK, Yang W, Tu JY. Simulation of sprayed particle deposition in a human nasal cavity including a nasal spray device. J Aerosol Sci. 2011;42(2):100–13.
Wen J, Inthavong K, Tu J, Wang S. Numerical simulations for detailed airflow dynamics in a human nasal cavity. Respir Physiol Neurobiol. 2008;161(2):125–35.
Schreck S, Sullivan KJ, Ho CM, Chang HK. Correlations between flow resistance and geometry in a model of the human nose. J Appl Physiol. 1993;75(4):1767–75.
Subramaniam RP, Richardson RB, Morgan KT, Kimbell JS, Guilmette RA. Computational fluid dynamics simulations of inspiratory airflow in the human nose and nasopharynx. Inhal Toxicol. 1998;10(2):91–120.
Beck-Broichsitter M, Knuedeler MC, Seeger W, Schmehl T. Controlling the droplet size of formulations nebulized by vibrating-membrane technology. Eur J Pharm Biopharm. 2014;87(3):524–9.
Gaspar MM, Gobbo O, Ehrhardt C. Generation of liposome aerosols with the Aeroneb Pro and the AeroProbe nebulizers. J Liposome Res. 2010;20(1):55–61.
Hertel S, Pohl T, Friess W, Winter G. Prediction of protein degradation during vibrating mesh nebulization via a high throughput screening method. Eur J Pharm Biopharm. 2014;87(2):386–94.
Xi J, Si XA, Gaide R. Electrophoretic particle guidance significantly enhances olfactory drug delivery: a feasibility study. PLoS One. 2014;9(1):1–11.
Golshahi L, Longest PW, Holbrook L, Snead J, Hindle M. Production of highly charged pharmaceutical aerosols using a new aerosol induction charger. Pharm Res. 2015;32(9):3007–17.
Hoekman JD, Ho RJY. Enhanced analgesic responses after preferential delivery of morphine and fentanyl to the olfactory epithelium in rats. Anesth Analg. 2011;113(3):641–51.
De Wang Y, Lee HP, Gordon BR. Impacts of fluid dynamics simulation in study of nasal airflow physiology and pathophysiology in realistic human three-dimensional nose models. Clin Exp Otorhinolaryngol. 2012;5(4):181–7.
Kim SK, Na Y, Kim JI, Chung SK. Patient specific CFD models of nasal airflow: overview of methods and challenges. J Biomech. 2013;46(2):299–306.
Kleven M, Melaaen MC, Reimers M, Røtnes JS, Aurdal L, Djupesland PG. Using computational fluid dynamics (CFD) to improve the bi-directional nasal drug delivery concept. Food Bioprod Process. 2005;83(2):107–17.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by Ulm and Biberach joint graduate school in pharmaceutical biotechnology funded by the Baden-Württemberg State Ministry of Science, Research and Arts. Special thanks go to Dr. Andreas Lintermann for his helpful support and valuable scientific comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lucas Engelhardt and Martina Röhm contributed equally to the manuscript and share first authorship.
Rights and permissions
About this article
Cite this article
Engelhardt, L., Röhm, M., Mavoungou, C. et al. First Steps to Develop and Validate a CFPD Model in Order to Support the Design of Nose-to-Brain Delivered Biopharmaceuticals. Pharm Res 33, 1337–1350 (2016). https://doi.org/10.1007/s11095-016-1875-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1875-7