Abstract
Purpose
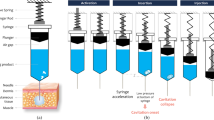
Interface motion and hydrodynamic shear of the liquid slosh during the insertion of syringes upon autoinjector activation may damage the protein drug molecules. Experimentally validated computational fluid dynamics simulations are used in this study to investigate the interfacial motion and hydrodynamic shear due to acceleration and deceleration of syringes. The goal is to explore the role of fluid viscosity, air gap size, syringe acceleration, syringe tilt angle, liquid-wall contact angle, surface tension and fill volume on the interface dynamics caused by autoinjector activation.
Methods
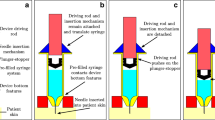
A simplified autoinjector platform submerged in water is built to record the syringe and liquid motion without obstruction of view. The syringe kinematics is imported to the simulations based on OpenFOAM InterIsoFoam solver, which is used to study the effects of various physical parameters.
Results
The simulations agree with experiments on the air-liquid interface profile and interface area. The interfacial area and the volume of fluid subject to high strain rate decrease with the solution viscosity, increase with the air gap height, syringe velocity, tilt angle and syringe wall hydrophobicity, and hardly change with the surface tension and liquid column height. The hydrodynamic shear mainly occurs near the syringe wall and entrained bubbles.
Conclusion
For a given dose of drug solution, the syringe with smaller radius and larger length will generate less liquid slosh. Reducing the air volume and syringe wall hydrophobicity are also helpful to reduce interface area and effective shear. The interface motion is reduced when the syringe axis is aligned with the gravitational direction.

















Similar content being viewed by others
Abbreviations
- CFD:
-
Computational Fluid Dynamics
- CFL:
-
Courant-Friedrichs-Lewy number
- mAb:
-
Monoclonal antibody
- SQ:
-
Subcutaneous
References
Mathaes R, Koulov A, Joerg S, Mahler HC. Subcutaneous injection volume of biopharmaceuticals—pushing the boundaries. J Pharm Sci. 2016;105(8):2255–9.
Yang MX, Shenoy B, Disttler M, Patel R, McGrath M, Pechenov S, et al. Crystalline monoclonal antibodies for subcutaneous delivery. Proc Natl Acad Sci. 2003;100(12):6934–9.
Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8(3):E501–7.
Frei PC, Benacerraf B, Thorbecke GJ. Phagocytosis of the antigen, a crucial step in the induction of the primary response. Proc Natl Acad Sci USA. 1965;1:20.
Moussa EM, Panchal JP, Moorthy BS, Blum JS, Joubert MK, Narhi LO, et al. Immunogenicity of therapeutic protein aggregates. J Pharma Sci. 2016;105(2):417–30.
Braun A, Kwee L, Labow MA, Alsenz J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-α) in normal and transgenic mice. Pharm Res. 1997;14(10):1472–8.
Kiese S, Papppenberger A, Friess W, Mahler HC. Shaken, not stirred: mechanical stress testing of an IgG1 antibody. J Pharm Sci. 2008;97(10):4347–66.
Thomas CR, Geer D. Effects of shear on proteins in solution. Biotechnol Lett. 2011;33(3):443–56.
Bee JS, Stevenson JL, Mehta B, Svitel J, Pollastrini J, Platz R, et al. Response of a concentrated monoclonal antibody formulation to high shear. Biotechnol Bioeng. 2009;103(5):936–43.
Mahler HC, Müller R, Frieβ W, Delille A, Matheus S. Induction and analysis of aggregates in a liquid IgG1-antibody formulation. Eur J pharm Biopharm. 2005;59(3):407–17.
Bekard IB, Asimakis P, Bertolini J, Dunstan DE. The effects of shear flow on protein structure and function. Biopolymers. 2011;95(11):733–45.
Sluzky V, Klibanov AM, Langer R. Mechanism of insulin aggregation and stabilization in agitated aqueous solutions. Biotechnol bioeng. 1992;40(8):895–903.
Bee JS, Nelson SA, Freund E, Carpenter JF, Randolph TW. Precipitation of a monoclonal antibody by soluble tungsten. J pharm sci. 2009;98(9):3290–301.
Baldascini H, Janssen DB. Interfacial inactivation of epoxide hydrolase in a two-liquid-phase system. Enzyme Microb Technol. 2005;36(2–3):285–93.
Dobson J, Kumar A, Willis LF, Tuma R, Higazi DR, Turner R, et al. Inducing protein aggregation by extensional flow. Proc Natil Acad Sci. 2017;114(18):4673–8.
Xing L, Li Y, Li T, Concentrating L. Not Shear Stress, That May Lead to Possible Instability of Protein Molecules During Syringe Injection: A Fluid Dynamic Study with Two-Phase Flow Model. PDA J Pharm Sci Technol. 2019;73(3):260–75.
Lin GL, Pathak JA, Kim DH, Carlson M, Riguero V, Kim YJ, et al. Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions. Soft Matter. 2016;12(14):3293–302.
Brych SR, Gokarn YR, Hultgen H, Stevenson RJ, Rajan R, Matsumura M. Characterization of antibody aggregation: role of buried, unpaired cysteines in particle formation. J Pharm Sci. 2010;99(2):764–81.
Zhong X, Guo T, Vlachos P, Veilleux JC, Shi GH, Collins DS, Ardekani AM. An experimentally validated dynamic model for spring-driven autoinjectors. Int J Pharm. 2021;594:120008.
Roenby J, Bredmose H. Jasak H. A computational method for sharp interface advection. R Soc Open Sci. 2016;3(11):160405.
Mead-Hunter R, King AJ, Mullins BJ. Plateau Rayleigh instability simulation. Langmuir. 2012;28(17):6731–5.
Raeini AQ, Blunt MJ, Bijeljic B. Modelling two-phase flow in porous media at the pore scale using the volume-of-fluid method. J Comput Phys. 2012;231(17):5653–68.
Koukouvinis P, Gavaises M, Supponen O, Farhat M. Simulation of bubble expansion and collapse in the vicinity of a free surface. Phys Fluids. 2016;28(5):052103.
Amin S, Barnett GV, Pathak JA, Roberts CJ, Sarangapani PS. Protein aggregation, particle formation, characterization & rheology. Curr Opin Colloid Interface Sci. 2014;19(5):438–49.
Castellanos MM, Pathak JA, Colby RH. Both protein adsorption and aggregation contribute to shear yielding and viscosity increase in protein solutions. Soft Matter. 2014;10(1):122–31.
Sharma V, Jaishankar A, Wang YC, McKinley GH. Rheology of globular proteins: apparent yield stress, high shear rate viscosity and interfacial viscoelasticity of bovine serum albumin solutions. Soft Matt. 2011;7(11):5150–60.
Brackbill JU, Kothe DB. Zemach C. A continuum method for modeling surface tension. J Comput Phys. 1992;100(2):335–54.
Wen ZQ, Vance A, Vega F, Cao X, Eu B, Schulthesis R. Distribution of silicone oil in prefilled glass syringes probed with optical and spectroscopic methods. PDA J Pharm Sci Technol. 2009;63(2):149–58.
Eifert A, Paulssen D, Varanakkottu SN, Baier T, Hardt S. Simple fabrication of robust water-repellent surfaces with low contact-angle hysteresis based on impregnation. Adv Mater Interfaces. 2014;1(3):1300138.
Azarbayjani AF, Jouyban A, Chan SY. Impact of surface tension in pharmaceutical sciences. J Pharm Pharm Sci. 2009;12(2):218–28.
Ooi A, Martin J, Soria J. Chong MS. A study of the evolution and characteristics of the invariants of the velocity-gradient tensor in isotropic turbulence. J Fluid Mech. 1999;381:141–74.
Hawe A, Wiggenhorn M, van de Weert M, Garbe JH, Mahler HC, Jiskoot W. Forced degradation of therapeutic proteins. J Pharm Sci. 2012;101(3):895–913.
Nauman JV, Campbell PG, Lanni F, Anderson JL. Diffusion of insulin-like growth factor I and ribonuclease through fibrin gels. Biophys J. 2007;92(12):4444–50.
Veilleux JC, Shepherd JE. Impulsive motion in a cylindrical fluid-filled tube terminated by a converging section. J Press Vessel Technol. 2019;141(2):021302.
Zhang Y, Guo T, Vlachos P, Ardekani AM. Velocity scaling and breakup criteria for jets formed due to acceleration and deceleration process. Phys Rev Fluids. 2020;5(7):074003.
Dandekar R, Ardekani AM. Monoclonal Antibody aggregation near silicone oil-water interfaces. Langmuir. 2021;37(4):1386–98.
Acknowledgments
This research was supported by a grant from the Eli Lilly and Company. Y. Z. acknowledges the support of Frederick N. Andrews Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1404 kb)
ESM 2
(MP4 649 kb)
ESM 3
(MP4 553 kb)
ESM 4
(MP4 628 kb)
ESM 5
(MP4 1536 kb)
ESM 6
(MP4 392 kb)
ESM 7
(MP4 447 kb)
ESM 8
(MP4 374 kb)
ESM 9
(MP4 576 kb)
ESM 10
(MP4 607 kb)
ESM 11
(MP4 227 kb)
ESM 12
(MP4 847 kb)
ESM 13
(MP4 923 kb)
ESM 14
(MP4 1421 kb)
ESM 15
(MP4 368 kb)
ESM 16
(MP4 297 kb)
ESM 17
(MP4 539 kb)
ESM 18
(MP4 448 kb)
ESM 19
(MP4 4855 kb)
ESM 20
(MP4 4753 kb)
ESM 21
(MP4 5251 kb)
ESM 22
(MP4 7038 kb)
ESM 23
(MP4 7932 kb)
ESM 24
(MP4 1953 kb)
ESM 25
(MP4 1204 kb)
ESM 26
(MP4 723 kb)
ESM 27
(MP4 378 kb)
ESM 28
(MP4 306 kb)
ESM 29
(M4V 2327 kb)
ESM 30
(M4V 1874 kb)
ESM 31
(M4V 1313 kb)
ESM 32
(M4V 1260 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Han, D., Dou, Z. et al. The Interface Motion and Hydrodynamic Shear of the Liquid Slosh in Syringes. Pharm Res 38, 257–275 (2021). https://doi.org/10.1007/s11095-021-02992-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-02992-3