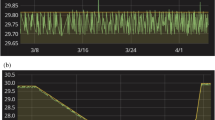
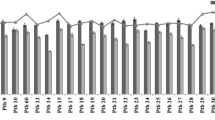
Abstract
Background
Fluctuating soil moisture resulting from the transient occurrences of waterlogging and drought are frequently reoccurring in the rice field, which adversely affects plant growth and yield. We previously established the significant contribution of plastic development and associated physiological responses of root to shoot dry matter production under soil moisture fluctuation stresses.
Aim
To evaluated the functional roles of root plastic development on yield under field condition of continuous cycle of transient soil moisture stresses.
Methods
Previously selected CSSL47 and the recurrent parent Nipponbare were exposed to two soil moisture conditions; cycles of alternating waterlogging and drought condition (CAW-D) and continuous waterlogging (CWL; control).
Results
Under continuous waterlogging (CWL) conditions, the two genotypes showed no significant differences in most of the traits examined. In contrast, under continuous cycle of alternate waterlogging and drought (CAW-D) conditions, CSSL47 showed greater shoot dry matter production than Nipponbare, which was attributed to its higher stomatal conductance and photosynthetic rate, which then led to higher grain yield. The root system development of CSSL47 expressed as total root length was greater compared with Nipponbare. Before heading stage, plasticity was expressed as enhanced aerenchyma formation based on root porosity, which was associated with the promotion of lateral root production, elongation and branching and the eventual increase in total root length. Moreover, after heading, compared with Nipponbare, CSSL47 continued to produce more nodal roots from newly produced tillers, thus maintaining leaf photosynthesis and eventually resulting in heavier panicles.
Conclusions
We provide evidences that root plasticity, which better expressed in CSSL47 than Nipponbare, under continuous cycle of transient soil moisture stresses contributed to increase in grain yield in fields. Genetic variation in plastic responses of roots could have substantial impact on yield in areas experiencing these kind of soil moisture stresses.







Similar content being viewed by others
Abbreviations
- CSSL:
-
Chromosome segment substituted line
- DAS:
-
Days after sowing
- CWL:
-
Continuous waterlogging
- CAW-D:
-
Continuous cycle of alternate waterlogging and drought
- SRL:
-
Specific root length
- TRL:
-
Total root length
References
Armstrong W (1971) Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration, and waterlogging. Physiol Plantarum 25:192–197
Azhiri-Sigari T, Yamauchi A, Kamoshita A, Wade LJ (2000) Genotypic variation in response of rainfed lowland rice to drought and rewatering. II. Root growth. Plant Prod Sci 3:180–188
Bañoc DM, Yamauchi A, Kamoshita A, Wade LJ, Pardales JR Jr (2000) Genotypic variations in response of lateral root development to fluctuating soil moisture in rice. Plant Prod Sci 3:335–343
Belder P, Bouman BAM, Cabangon R, Guoan Lu, Quilang EJP, Li Y, Spiertz JHJ, Tuong TP (2004) Effect of water-saving irrigation on rice yield and water use in typical lowland conditions in Asia. Agr Water Manag 65:193–210
Belder P, Bouman BAM, Spiertz JHJ, Peng S, Castañeda AR, Visperas RM (2005) Crop performance, nitrogen and water use in flooded and aerobic rice. Plant Soil 273:167–182
Boling A, Toung TP, Jatmiko SY, Burac MA (2004) Yield constraints of rainfed lowland rice in Central Java, Indonesia. Field Crops Res 90:351–360
Bouman BAM, Peng S, Castañeda AR, Visperas RM (2005) Yield yield and water use of irrigated tropical aerobic rice. Agr Water Manag 74:87–105
Champoux MC, Wang G, Sarkarung S, Mackill DJ, O’Toole JC, Huang N, MacCouch SR (1995) Locating genes associated with root morphology and drought avoidance in rice via linkage to molecular markers. Theor Appl Gene 90:969–981
Colmer TD (2003) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deepwater rice (Oryza sativa L.). Ann Bot 91:301–309
Eissentat D (1991) On the relationship between specific root length and the rate of root proliferation: a field study using citrus rootstocks. New Phytol 118:63–68
Gowda VRP, Henry A, Yamauchi A, Shashidhar HE, Serraj R (2011) Root biology and genetic improvement for drought avoidance in rice. Field Crops Res 122:1–13
Henry A, Gowda VRP, Torres R, McNally K, Serraj R (2011) Genetic variation in root architecture and drought response in Oryza sativa: Rainfed lowland field studies of the OryzaSNP panel. Field Crops Res 120:205–214
Horii H, Nemoto K, Miyamoto N, Harada J (2006) Quantitative trait loci for adventitious and lateral roots in rice. Plant Breed 125:198–200
Iijima M, Morita S, Zegada-Lizarazu W, Izumi Y (2007) No-tillage enhanced the dependence on surface irrigation water in wheat and soybean. Plant Prod Sci 10:182–188
Ito K, Tanakamaru K, Morita S, Abe J, Inanaga S (2006) Lateral root development, including responses to soil drying, of maize (Zea mays) and wheat (Triticum aestivum) seminal roots. Physiol Plant 127:260–267
Kamoshita A, Wade LJ, Yamauchi A (2000) Genotypic variation in response of rainfed lowland rice to drought and rewatering III. Water extraction during the drought period. Plant Prod Sci 3:189–196
Kamoshita A, Rodriquez R, Yamauchi A, Wade LJ (2004) Genotypic variation in response of rainfed lowland rice to prolong drought and rewatering. Plant Prod Sci 7:406–420
Kang SY, Morita S, Yamazaki K (1994) Root growth and distribution in some japonica-indica hybrid and Japonica type rice cultivars under field conditions. Jpn J Crop Sci 63:118–124
Kano M, Inukai Y, Kitano H, Yamauchi A (2011) Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil 342:117–128
Kano-Nakata M, Inukai Y, Wade LJ, Siopongco JDLC, Yamauchi A (2011) Root development and water uptake, and shoot dry matter production under water deficit conditions in two CSSLs of rice: functional roles of root plasticity. Plant Prod Sci 14:307–317
Kato Y, Kamoshita A, Yamagishi J, Imoto H, Abe J (2007) Growth of rice (Oryza sativa L.) cultivars under upland conditions with different levels of water supply. Plant Prod Sci 10:3–13
Kato Y, Henry A, Fujita D, Katsura K, Kobayashi N, Serraj R (2011) Physiological characterization of introgression lines derived from an indica rice cultivar, IR64, adapted to drought and water-saving irrigation. Field Crops Res 123:130–138
Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35:25–34
Kimura K, Yamasaki S (2001) Root length and diameter measurement using NIH Image: application of the line-intercept principle for diameter estimation. Plant Soil 234:37–46
Kimura K, Kikuchi S, Yamasaki S (1999) Accurate root length measurement by image analysis. Plant Soil 216:117–127
Kobata T, Okuno T, Yamamoto T (1996) Contribution of capacity for soil water extraction and water use efficiency to maintenance of dry matter production in rice subjected to drought. Jpn J Crop Sci 65:652–662
Kondo M, Sing CV, Agbisit R, Murty MVR (2005) Yield response to urea and controlled-release urea as affected by water supply in tropical upland rice. J Plant Nutr 28:201–219
Kono Y, Tomida K, Tatsumi J, Nonoyama T, Yamauchi A, Kitano J (1987) Effects of soil moisture conditions on the development of root systems of soybean plants (Glycine max Merr.). Jpn J Crop Sci 56:597–607
Macmillan K, Emrich K, Piepho HP, Mullins CE, Price AH (2006) Assessing the importance of genotype X environment interaction for root traits in rice using a mapping population II: conventional QTL analysis. Theor Appl Genet 113:953–964
Miyamoto N, Steudle E, Hirasawa T, Lafitte R (2001) Hydraulic conductivity of rice roots. J Exp Bot 52:1835–1846
O’Toole JC, Bland WL (1987) Genotypic variation in crop plant root systems. Adv Agron 41:91–145
Pardales JR Jr, Yamauchi A (2003) Regulation of root development in sweet potato and cassava by soil moisture during their establishment period. Plant Soil 255:201–208
Price AH, Steele KA, Moore BJ, Jones RGW (2002) Upland rice grown in soil filled chambers and exposed to contrasting water-deficit regimes II. Mapping quantitative trait loci for root morphology and distribution. Field Crop Res 76:25–43
Raumet C, Urcelay C, Diaz S (2006) Suites of root traits differ between annual and perennial species growing in the field. New Phytol 170:357–368
Schippers P, Olff H (2000) Biomass partitioning, architecture and turnover of six herbaceous species from habitats with different nutrient supply. Plant Ecol 149:219–231
Siopongco JDLC, Yamauchi A, Salekdeh H, Bennett J, Wade LJ (2005) Root growth and water extraction responses of double haploid rice lines to drought and rewatering during the vegetative stage. Plant Prod Sci 9:141–151
Siopongco JDLC, Yamauchi A, Salekdeh H, Bennett J, Wade LJ (2006) Growth and water use response of doubled haploid rice lines to drought and rewatering during the vegetative stage. Plant Prod Sci 9:141–151
Siopongco JDLC, Sekiya K, Yamauchi A, Egdane J, Ismail AM, Wade LJ (2008) Stomatal responses in rainfed lowland rice to partial soil drying; evidence of root signals. Plant Prod Sci 11:28–41
Siopongco JDLC, Sekiya K, Yamauchi A, Egdane J, Ismail AM, Wade LJ (2009) Stomatal responses in rainfed lowland rice to partial soil drying; comparison of two lines. Plant Prod Sci 12:17–28
Subere JOQ, Bolatete D, Bergatin R, Pardales A, Belmonte JJ, Mariscal A, Sebidos R, Yamauchi A (2009) Genotypic variation in tesponse of cassava (Manihot esculenta Crantz) to drought and rewatering. I. Root system development. Plant Prod Sci 12:462–474
Suga S, Murai M, Kuwagata T, Maeshima M (2003) Differences in aquaporin levels among cell types of radish and measurement of osmotic water permeability of individual protoplast. Plant Cell Physiol 44:277–286
Suralta RR (2011) Plastic root system development responses to drought-Enhanced nitrogen uptake during progressive soil drying conditions in rice. Philippine Agr Sci 93:458–462
Suralta RR, Yamauchi A (2008) Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging. Environ Exp Bot 64:75–82
Suralta RR, Inukai Y, Yamauchi A (2008a) Genotypic variations in responses of lateral root development to transient moisture stresses in rice cultivars. Plant Prod Sci 11:324–335
Suralta RR, Inukai Y, Yamauchi A (2008b) Utilizing chromosome segment substitution lines (CSSLs) for evaluation of root responses under transient moisture stresses in rice. Plant Prod Sci 11:457–465
Suralta RR, Inukai Y, Yamauchi A (2010) Dry matter production in relation to root plastic development. Oxygen transport, and water upatake of rice under transient soil moisture stresses. Plant Soil 332:87–104
Tabbal DF, Bouman BAM, Bhuiyan SI, Sibayan EB, Sattar MA (2002) On-farm strategies for reducing water input in irrigated rice; case studies in the Philippines. Agr Water Manag 56:93–112
Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167:493–508
Visser EJW, Bögenmann GM (2003) Measurement of porosity in very small samples of plant tissue. Plant Soil 253:81–90
Wade LJ, George T, Ladha JK, Singh U, Bhuiyan SI, Pandey S (1998) Opportunities to manipulate nutrient-by-water interactions in rainfed lowland rice systems. Field Crop Res 56:93–112
Wade LJ, Kamoshita A, Yamauchi A, Azhiri-Sigari T (2000) Genotypic variation in response of rainfed rowland rice to drought and rewatering I. Growth and water use. Plant Prod Sci 3:173–179
Wang H, Yamauchi A (2006) Growth and function of roots under abiotic stress soils. In: Huang B (ed) Plant-environment interactions, 3rd edn. CRC Press, Taylor and Francis Group, LLC, New York, pp 271–320
Wang H, Siopongco JDLC, Wade LJ, Yamauchi A (2009) Fractal analysis on root systems of rice plants in response to drought stress. Environ Exp Bot 65:338–344
Yadav S, Humphreys E, Kukal SS, Gill G, Rangarajan R (2011) Effect of water management on dry seeded and puddle transplanted rice Part 2: Water balance and water productivity. Field Crop Res 120:123–132
Yamauchi A, Kono Y, Tatsumi J (1987) Quantitative analysis on root system structure of upland rice and maize. Jpn J Crop Sci 56:608–617
Yamauchi A, Pardales JR Jr, Kono Y (1996) Root system structure and its relation to stress tolerance. In: Ito O, Katayama K, Johansen C, Kumar Rao JVDK, Adu-Gyamfi JJ, Rego TJ (eds) Roots and nitrogen in cropping systems of the semi-arid tropics. JIRCAS Publication, Tsukuba, Japan, pp 211–234
Yambao EB, Ingram KT, Real JG (1992) Root xylem influence on the water relations and drought resistance of rice. J Exp Bot 43:925–932
Yoshida S (1981) Fundamentals of rice crop science. The International Rice Research Institute, Los Baños, Laguna, Manila, Philippines, 269 p
Zhang WP, Shen XY, Wu P, Hu B, Liao CY (2001) QTLs and epistasis for seminal root length under a different water supply in rice (Oryza Sativa L.). Theor Appl Genet 103:118–123
Zheng BS, Yang L, Zhang WP, Mao CZ, Wu YR, Yi KK, Liu FY, Wu P (2003) Mapping QTLs and candidate genes for rice root traits under different water-supply conditions and comparative analysis across three populations. Theor Appl Genet 107:1505–1515
Zheng BS, Yang L, Zhang WP, Mao CZ, Wu P (2006) Comparison of QTLs for rice root morphology under different water supply conditions. Acta Genetica Sinica 33:141–151
Acknowledgement
We thank Dr. Abdelbagi M. Ismail of International Rice Research Institute for his critical review and useful comments on our manuscript. This research was supported by Grant-Aid for Scientific Research (No. 22380013) from the Japan Society for the Promotion of Science and contributed to the Generation Challenge Project under the Consultative Group for International Agricultural Research on “Targeting drought avoidance root traits to enhance rice productivity under water-limited environments”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter J. Gregory.
Rights and permissions
About this article
Cite this article
Niones, J.M., Suralta, R.R., Inukai, Y. et al. Field evaluation on functional roles of root plastic responses on dry matter production and grain yield of rice under cycles of transient soil moisture stresses using chromosome segment substitution lines. Plant Soil 359, 107–120 (2012). https://doi.org/10.1007/s11104-012-1178-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1178-7