Abstract
Aims
This study explored a possibility that leaf silicious trichomes slow down leaf mass loss by soil meso- and macrofauna.
Methods
We focused on closely related Moraceae tree species (Broussonetia papyrifera and Morus australis) with different leaf trichome densities. First, we conducted microscopic analyses of leaf trichomes and investigated nine other leaf traits. We then performed a 25-day decomposition experiment with bags of different mesh sizes that excluded and included meso- and macrofauna. A cafeteria experiment was also implemented to examine palatability of leaf powder (i.e. trichome effects removed) to the most abundant macrofauna (Armadillidium vulgare) in our decomposition site.
Results
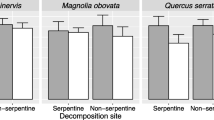
Broussonetia papyrifera exhibited a 150-fold higher silicious trichome density on the lower leaf surface than M. australis. The carbon-to-nitrogen ratio was significantly lower in B. papyrifera than M. australis. In decomposition, the ash-free mass loss of B. papyrifera was significantly higher than that of M. australis in the <0.2-mm mesh bag. Similarly, in the cafeteria experiment with A. vulgare, mass loss of B. papyrifera leaf powder was significantly higher than that of M. australis. In the 5-mm mesh bag, however, the ash-free mass loss of B. papyrifera was significantly lower than that of M. australis.
Conclusions
Despite rich nutrient quality of B. papyrifera leaves, leaf decomposition of B. papyrifera with greater trichome densities was slower than that of M. australis only in the 5-mm mesh bag that permitted the entry of meso- and macrofauna. This suggests a suppressive effect of leaf silicious trichomes on decomposition by the large decomposers.



Similar content being viewed by others
Data availability
Data available on request.
Code availability
Not applicable.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 79:439–449
Agren J, Schemske DW (1993) The cost of defense against herbivores: an experimental study of trichome production in Brassica rapa. Am Nat 141:338–350
Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press
Barthlott W, Schimmel T, Wiersch S, Koch K, Brede M, Barczewski M, Walheim S, Weis A, Kaltenmaier A, Leder A (2010) The Salvinia paradox: Superhydrophobic surfaces with hydrophilic pins for air retention under water. Adv Mater 22:2325–2328
Bickford CP (2016) Ecophysiology of leaf trichomes. Funct Plant Biol 43:807–814
Birch H (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10:9–31
Boeger MRT, Alves LC, Negrelle RRB (2004) Leaf morphology of 89 tree species from a lowland tropical rain forest (Atlantic forest) in South Brazil. Braz Arch Biol Technol 47:933–943
Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE (2002) Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99:317–323
Bray SR, Kitajima K, Mack MC (2012) Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol Biochem 49:30–37
Brewer CA, Smith WK, Vogelmann TC (1991) Functional interaction between leaf trichomes, leaf wettability and the optical-properties of water droplets. Plant Cell Environ 14:955–962
Broadhurst CL, Chaney RL, Angle JS, Maugel TK, Erbe EF, Murphy CA (2004) Simultaneous hyperaccumulation of nickel, manganese, and calcium in Alyssum leaf trichomes. Environ Sci Technol 38:5797–5802
Conley DJ, Schelske CL (1993) Potential role of sponge spicules in influencing the silicon biogeochemistry of Florida Lakes. Can J Fish Aquat Sci 50:296–302
Cornelissen J (1996) An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol:573–582
Cornelissen JHC, Thompson K (1997) Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytol 135:109–114
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cortez J (1998) Field decomposition of leaf litters: relationships between decomposition rates and soil moisture, soil temperature and earthworm activity. Soil Biol Biochem 30:783–793
Dalin P, Ågren J, Björkman C, Huttunen P, Kärkkäinen K (2008) Leaf trichome formation and plant resistance to herbivory. In: S A. (ed) Induced plant resistance to herbivory. Springer, Dordrecht
eFloras (2008) Published on the Internet http://www.efloras.org [accessed 11 December 2020] Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA
Ehleringer J, Bjorkman O, Mooney HA (1976) Leaf pubescence - effects on absorptance and photosynthesis in a desert shrub. Science 192:376–377
Freschet GT, Aerts R, Cornelissen JHC (2012) A plant economics spectrum of litter decomposability. Funct Ecol 26:56–65
Frouz J, Roubickova A, Hedenec P, Tajovsky K (2015) Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. Eur J Soil Biol 68:18–24
Garcia-Palacios P, Maestre FT, Kattge J, Wall DH (2013) Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett 16:1045–1053
Gergocs V, Hufnagel L (2016) The effect of microarthropods on litter decomposition depends on litter quality. Eur J Soil Biol 75:24–30
Gilbert LE (1971) Butterfly-plant coevolution - has Passiflora Adenopoda won selectional race with heliconiine butterflies. Science 172:585–586
Gravuer K, Eskelinen A (2017) Nutrient and rainfall additions shift phylogenetically estimated traits of soil microbial communities. Front Microbiol 8
Gruner DS, Taylor AD, Forkner RE (2005) The effects of foliar pubescence and nutrient enrichment on arthropod communities of Metrosideros polymorpha (Myrtaceae). Ecol Entomol 30:428–443
Ichie T, Inoue Y, Takahashi N, Kamiya K, Kenzo T (2016) Ecological distribution of leaf stomata and trichomes among tree species in a Malaysian lowland tropical rain forest. J Plant Res 129:625–635
Johnson HB (1975) Plant pubescence - ecological perspective. Bot Rev 41:233–258
Korndörfer AP, Del-Claro K (2006) Ant defense versus induced defense in Lafoensia pacari (Lythraceae), a myrmecophilous tree of the Brazilian Cerrado. Biotropica 38:786–788
Kurokawa H, Nakashizuka T (2008) Leaf herbivory and decomposability in a Malaysian tropical rain forest. Ecology 89:2645–2656
Leff JW, Jones SE, Prober SM, Barberan A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JMH, McCulley RL, La Pierre K, Risch AC, Seabloom EW, Schutz M, Steenbock C, Stevens CJ, Fierer N (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. P Natl Acad Sci USA 112:10967–10972
Leroy N, de Tombeur F, Walgraffe Y, Cornelis JT, Verheggen FJ (2019) Silicon and plant natural defenses against insect pests: impact on plant volatile organic compounds and cascade effects on multitrophic interactions. Plants-Basel 8:444
Levin DA (1973) The role of trichomes in plant defense. Q Rev Biol 48:3–15
Lin DM, Wang F, Fanin N, Pang M, Dou PP, Wang HJ, Qian SH, Zhao L, Yang YC, Mi XC, Ma KP (2019) Soil fauna promote litter decomposition but do not alter the relationship between leaf economics spectrum and litter decomposability. Soil Biol Biochem 136
Marxen A, Klotzbucher T, Jahn R, Kaiser K, Nguyen VS, Schmidt A, Schadler M, Vetterlein D (2016) Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil 398:153–163
Massey FP, Ennos AR, Hartley SE (2007) Grasses and the resource availability hypothesis: the importance of silica-based defences. J Ecol 95:414–424
Matsuki S, Sano Y, Koike T (2004) Chemical and physical defence in early and late leaves in three heterophyllous birch species native to northern Japan. Ann Bot 93:141–147
Mattson WJ, Lawrence RK, Haack RA, Herms DA, Charles P-J (1988) Defensive strategies of woody plants against different insect-feeding guilds in relation to plant ecological strategies and intimacy of association with insects. In: Mattson LJ, W.J., Bernard-Dagan C. (eds) Mechanisms of woody plant defenses against insects. Springer, New York
Mauricio R (1998) Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana. Am Nat 151:20–28
Mori T, Lu XK, Aoyagi R, Mo JM (2018) Reconsidering the phosphorus limitation of soil microbial activity in tropical forests. Funct Ecol 32:1145–1154
Nakamura R, Cornelis JT, de Tombeur F, Nakagawa M, Kitajima K (2020a) Comparative analysis of borate fusion versus sodium carbonate extraction for quantification of silicon contents in plants. J Plant Res 133:271–277
Nakamura R, Cornelis JT, de Tombeur F, Yoshinaga A, Nakagawa M, Kitajima K (2020b) Diversity of silicon release rates among tropical tree species during leaf-litter decomposition. Geoderma 368:114288
Orgiazzi A, Bardgett RD, Barrios E (2016) Global soil biodiversity atlas. European Commission
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Petersen H, Luxton M (1982) A comparative-analysis of soil fauna populations and their role in decomposition processes. Oikos 39:287–388
Pillemer EA, Tingey WM (1976) Hooked trichomes - physical plant barrier to a major agricultural pest. Science 193:482–484
Piperno DR (2006) Phytoliths: a comprehensive guide for archaeologists and paleoecologists. Rowman Altamira
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Pyykkö M (1979) Morphology and anatomy of leaves from some woody plants in a humid tropical forest of Venezuelan Guayana. Acta Bot Fenn 112:1–41
R Core Team (2020) R: A language an environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reis F, Nascimento E, Castro H, Canhoto C, Goncalves AL, Simoes S, Garcia-Palacios P, Milla R, Sousa JP, da Silva PM (2018) Land management impacts on the feeding preferences of the woodlouse Porcellio dilatatus (Isopoda: Oniscidea) via changes in plant litter quality. Appl Soil Ecol 132:45–52
Rosenfield MV, Keller JK, Clausen C, Cyphers K, Funk JL (2020) Leaf traits can be used to predict rates of litter decomposition. Oikos 129:1589–1596
Roth I (1984) Stratification of tropical forests as seen in leaf structure. Den Haag, Dr. W. Junk Publishers (Tasks for vegetation science)
Schaller J (2013) Invertebrate grazers are a crucial factor for grass litter mass loss and nutrient mobilization during aquatic decomposition. Fund Appl Limnol 183:287–295
Schaller J, Struyf E (2013) Silicon controls microbial decay and nutrient release of grass litter during aquatic decomposition. Hydrobiologia 709:201–212
Schaller J, Hines J, Brackhage C, Baucker E, Gessner MO (2014) Silica decouples fungal growth and litter decomposition without changing responses to climate warming and N enrichment. Ecology 95:3181–3189
Schlesinger WH, Bernhardt ES (2013) Biogeochemistry: an analysis of global change. Academic press
Smith VC, Bradford MA (2003) Litter quality impacts on grassland litter decomposition are differently dependent on soil fauna across time. Appl Soil Ecol 24:197–203
Valverde P, Fornoni J, Núñez-Farfán J (2001) Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. J Evol Biol 14:424–432
Van Soest PJ (1963) Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J Assoc Off Agric Chem 46:829–835
Waterman JM, Cibils-Stewart X, Cazzonelli CI, Hartley SE, Johnson SN (2021) Short-term exposure to silicon rapidly enhances plant resistance to herbivory. In press
Werker E (2000) Trichome diversity and development. Adv Bot Res 31:1–35
Xu X, Sun Y, Sun JJ, Cao PH, Wang YC, Chen HYH, Wang WF, Ruan HH (2020) Cellulose dominantly affects soil fauna in the decomposition of forest litter: A meta-analysis. Geoderma 378
Yang XD, Chen J (2009) Plant litter quality influences the contribution of soil fauna to litter decomposition in humid tropical forests, southwestern China. Soil Biol Biochem 41:910–918
Zidar P, Kos M, Ilic E, Marolt G, Drobne D, Kokalj AJ (2019) Avoidance behaviour of isopods (Porcellio scaber) exposed to food or soil contaminated with ag- and CeO2-nanoparticles. Appl Soil Ecol 141:69–78
Acknowledgements
We thank Saori Fujii, Michimasa Yamasaki and members of the Kitajima laboratory for their constructive comments on this research. We appreciate Yusuke Onoda and the staff at the Kitashirakawa Experimental Station for their experimental support. We thank Naoto Nakamura for pre-submission review. This research was financially supported by the Research Institute of Sustainable Humanosphere, Kyoto University.
Funding
Research Institute of Sustainable Humanosphere, Kyoto University.
Author information
Authors and Affiliations
Contributions
RN, GA and HK conceived the ideas and designed methodology; RN, GA, HK, KM, MH collected the data; RN, GA and HK analyzed the data; RN led the writing of the manuscript; HK, GA, KK and HK revised it critically for important intellectual content. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/Competing interests
We declare no conflict of interests.
Additional information
Responsible Editor: Martin J. Hodson
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 8013 kb)
Rights and permissions
About this article
Cite this article
Nakamura, R., Amada, G., Kajino, H. et al. Silicious trichomes as a trait that may slow down leaf decomposition by soil meso- and macrofauna. Plant Soil 471, 289–299 (2022). https://doi.org/10.1007/s11104-021-05223-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05223-1