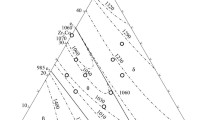
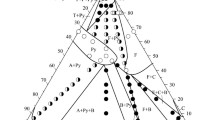
The phase equilibria in the Zr–ZrCo–ZrNi system at subsolidus temperature and at 900 and 800°C were first studied using physicochemical analysis methods, and solidus surfaces and isothermal sections at 900 and 800°C were constructed. Isomorphic Zr2Co and Zr2Ni compounds with a tetragonal crystal structure of AlCu2 (θ) type form a continuous series of solid solutions dividing the Zr–ZrCo–ZrNi system into two subsystems: Zr–Zr2Co–Zr2Ni and ZrCo–ZrNi–Zr2Ni–Zr2Co. The equilibria on the solidus surface of the Zr–Zr2Co–ZrCo–Zr2Ni system and at 900°C differ significantly. This is associated with the Zr3Co-based η phase formed by peritectoid reaction 〈β − Zr〉 + 〈Zr2Co〉 → η at 980°C, being close to theL ↔ θ + β eutectic crystallization temperature (986°C), in the binary Zr–Co system. At 900 and 800°C, the 𝜂 phase dissolves up to 14.5% Ni. The solidus surface of the ternary Zr2Co–Zr2Ni–ZrCo–ZrNi system shows a three-phase equilibrium of the θ phase with the ZrCo (δ) and ZrNi (δ2) phases of the ZrCo–ZrNi quasibinary section: δ + δ2 + θ. The plane of this tie-line triangle extends significantly at 900 and 800°C as the solubility of nickel in the cubic ZrCo-based phase of CsCl type changes. At room temperature, all alloys of the ZrCo–ZrNi–Zr2Ni–Zr2Co subsystem should contain three phases: δ + δ2 + θ. The solidus surface of the Zr–ZrCo ZrNi system is thus completed by surfaces corresponding to the homogeneity regions of the δ, δ2, δ, and β phases, θ + δ + δ2 tie-line triangle plane, and ruled surfaces representing the upper boundary of the two-phase θ + δ2, θ + δ, and θ + δ volumes. At 900 and 800°C, two three-phase equilibria, η + θ + β and δ + δ2 + θ, are observed in the system.





Similar content being viewed by others
Notes
Composition is in at.% here and further in the text.
References
E.E. Novikova, Ye.V. Tatyanin, and V.G. Kurdjumov, “Peculiarities of deformation-induced amorphization in CoZrNi alloys,” Scr. Metall. Mater., 33, No. 6, 851–855 (1995).
A. Cziraki, F. Zhou, R. Luck, K. Lu, A. Lovas, and I. Bakonyi, “Formation and microstructure of nanocrystalline phases in Ni-rich melt-quenched Zr–Ni alloys,” Scr. Mater., 44, 1287–1290 (2001).
J.C. Sun, S. Li, and S.J. Li, “The effects of subsolution of Ti and La for Zr in ZrMn0.7V0.2Co0.1Ni1.2 hydrogen storage alloys on the phase structure and electrochemical properties,” J. Alloys Compd., 630, 446–447 (2007).
S. J. Pang, T. Zhang, K. Asaui, and A. Inoue, “Glassy NiTa–Ti–Zr(Co) alloys with high thermal stability,” Mater. Trans., 43, 1771 (2002).
T. Zhang, Sh. Pang, K. Asami, and A. Inoue, “Glassy Ni–Ta–Ti–Zr(Co) alloys with high thermal stability and high corrosion resistance,” Mater. Trans., 44, No. 11, 2322–2325 (2003).
J.Y. Huot, A. Van Neste, L. Brossard, and R. Schulz, “Amorphous Ni–Co–Zr alloys for water electrolysis in alkaline medium at 70°C,” Surf. Coat. Technol., 35, No. 3–4, 241–262 (1988).
K. Yamaya, T. Sambongi, and T. Mitsui, “Superconductivity and magnetic susceptibility of Zr2Co–Zr2Ni system,” J. Phys. Soc. Jpn., 29, No. 4, 879–884 (1970).
E.E. Havinga, H. Damsma, and P. Hokkeling, “Compounds and pseudobinary alloys with the CuAl2 (C16)-type structure. I. Preparation and X-ray results,” J. Less-Common Met., 27, 169–186 (1972).
V.G. Ivanchenko, T.O. Kosorukova, and V.V. Pogorila, “Study of phase equilibria in the Zr2Co–Zr2Ni system,” Metalloznav. Obrob. Met., 1, 19–22 (2004).
V.G. Ivanchenko and T.A. Kosorukova, “Phase equilibria in the ZrCo–ZrNi–Zr2Ni–Zr2Co partial system,” Chem. Met. Alloys, 1, No. 1, 73–75 (2008).
G. Zhou, Z. Liu, Z. Jin, and D. Zeng, “Phase equilibria of the Co–Ni–Zr system at 1198 K,” Mater. Lett., 64, No. 4, 549–551 (2010).
X. Liu, S. Yang, H. Xiong, W. Yu, Y. Cheng, H. Wu, and C. Wang, “Experimental investigation of phase equilibria in the Co–Ni–Zr ternary system,” Int. J. Mater. Res., 107, 1–7 (2016).
O.L. Semenova, V.M. Petyukh, and O.S. Fomichov, “The constitution of Co–Zr phase diagram,” Powder Metall. Met. Ceram., 54, No. 9–10, 583–589 (2016).
O.L. Semenova, V.M. Petyukh, and O.S. Fomichov, “Specification of elements in phase equilibria in the Zr–Ni system,” Proc. 20th Ukr. Conf. Inorganic Chemistry [in Ukrainian], (September 17–20, 2018, Dnipro, Ukraine), Dnipro (2018), p. 185.
O.L. Semenova, V.M. Petyukh, and O.S. Fomichov, “The quasibinary ZrCo–ZrNi phase diagram,” Powder Metall. Met. Ceram., 56, No. 3–4, 210–219 (2017).
N. Bochvar, O. Abdulov, T. Dobatkina, M. Kareva, and O. Semenova, Ni–Zr Binary Phase Diagram Evaluation edited by G. Effenberg, Materials Science International, Stuttgart (2015), Document ID: 20.11406.1.2.
P.I. Kripiakevich, V.Ya. Markiv, and V.V. Burnasheva, “Crystal structure of Zr3Co compound,” Dop. Akad. Nauk USSR, No. 6, 551–553 (1970).
D.M. Bailey and J.F. Smith, “A note on the structure of Zr2Co,” Acta Cryst., 14, 1084 (1961).
W.P. Pechin, D.E. Williams, and W.L. Larsen, “The zirconium–cobalt alloy system,” Trans. ASM, 57, 464–473 (1964).
M.E. Kirpatrick, J.F. Smith, and W.L. Larsen, “The structures of NiZr2, NiZr and their hafnium analogs,” Acta Cryst., 15, 252–255 (1962).
G. Nolze, “PowderCell: A mixture between crystal structure visualizer, simulation and refinement tool,” in: Proc. 2nd Int. School on Powder Diffraction (January 20–23, 2002, IACS, Kolkata, India), Kolkata (2002), pp. 146–155.
N. Wang, C. Li, Z. Du, and F. Wang, “Experimental study and thermodynamic reassessment of the Ni–Zr system,” Calphad, 31, 413–421 (2007).
J.K. Stalick, L.A. Bendersky, and R.M. Waterstrat, “One-dimensional disorder in Zr9M11 (M = Ni, Pd, Pt) and low-temperature atomic mobility in Zr9Ni11,” J. Phys. Condens. Matter, 20, 1–10 (2008).
J.L. Glimois, C. Becle, G. Develey, and J.M. Moreau, “Crystal structure of the intermetallic compound Ni11Zr9,” J. Less-Common Met., 64, No. 1, 87–90 (1979).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 59, Nos. 9–10 (535), pp. 101–114, 2020.
Rights and permissions
About this article
Cite this article
Semenova, O., Petyukh, V. & Fomichov, O. The Co–Ni–Zr Phase Diagram in the Zr–ZrCo–ZrNi Region I. Phase Equilibria in the Zr–ZrCo–ZrNi System at Subsolidus Temperature, 900°C, and 800°C. Powder Metall Met Ceram 59, 564–575 (2021). https://doi.org/10.1007/s11106-021-00186-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00186-5