Abstract
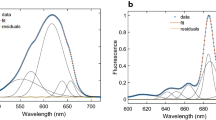
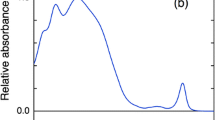
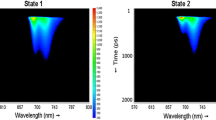
We prepared thylakoid membranes from Halomicronema hongdechloris cells grown under white fluorescent light or light from far-red (740 nm) light-emitting diodes, and observed their energy-transfer processes shortly after light excitation. Excitation–relaxation processes were examined by steady-state and time-resolved fluorescence spectroscopies. Two time-resolved fluorescence techniques were used: time-correlated single photon counting and fluorescence up-conversion methods. The thylakoids from the cells grown under white light contained chlorophyll (Chl) a of different energies, but were devoid of Chl f. At room temperature, the excitation energy was equilibrated among the Chl a pools with a time constant of 6.6 ps. Conversely, the thylakoids from the cells grown under far-red light possessed both Chl a and Chl f. Two energy-transfer pathways from Chl a to Chl f were identified with time constants of 1.3 and 5.0 ps, and the excitation energy was equilibrated between the Chl a and Chl f pools at room temperature. We also examined the energy-transfer pathways from phycobilisome to the two photosystems under white-light cultivation.






Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- DV-Chl:
-
3,8-Divinyl chlorophyll
- LED:
-
Light-emitting diodes
- PBS:
-
Phycobilisome
- PS:
-
Photosystem
- TRFS:
-
Time-resolved fluorescence spectrum (spectra)
References
Akimoto S, Yokono M, Ohmae M, Yamazaki I, Tanaka A, Higuchi M, Tsuchiya T, Miyashita H, Mimuro M (2004) Ultrafast excitation relaxation dynamics and energy transfer in the siphonaxanthin-containing green alga Codium fragile. Chem Phys Lett 390:45–49
Akimoto S, Yokono M, Ohmae M, Yamazaki I, Tanaka A, Higuchi M, Tsuchiya T, Miyashita H, Mimuro M (2005) Ultrafast excitation relaxation dynamics of lutein in solution and in the light-harvesting complexes II isolated from Arabidopsis thaliana. J Phys Chem B 109:12612–12619
Akimoto S, Yokono M, Hamada F, Teshigahara A, Aikawa S, Kondo A (2012) Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta 1817:1483–1489
Akimoto S, Teshigahara A, Yokono M, Mimuro M, Nagao R, Tomo T (2014) Excitation relaxation dynamics and energy transfer in fucoxanthin-chlorophyll a/c-protein complexes, probed by time-resolved fluorescence. Biochim Biophys Acta 1837:1514–1521
Boardman NK, Thome SW, Anderson JM (1966) Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts. Proc Natl Acad Sci USA 56:586–593
Chen M, Schliep M, Willows RD, Cai ZL, Neilan BA, Scheer H (2010) A red-shifted chlorophyll. Science 329:1318–1319
Chen M, Li YQ, Birch D, Willows RD (2012) A cyanobacterium that contains chlorophyll f - a red-absorbing photopigment. FEBS Lett 586:3249–3254
Connelly JP, Müller MG, Bassi R, Croce R, Holzwarth AR (1997) Femtosecond transient absorption study of carotenoid to chlorophyll energy transfer in the light-harvesting complex II of photosystem II. Biochemistry 36:281–287
Förster T (1948) Intermolecular energy migration and fluorescence. Ann Phys 2:55–75
Gan F, Zhang S, Rockwell NC, Martin SS, Lagarias JC, Bryant DA (2014) Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 345:1312–1317
Goedheer JC (1972) Fluorescence in relation to photosynthesis. Ann Rev Plant Physiol 23:87–112
Govindjee Satoh K (1986) Fluorescence properties of chlorophyll b- and chlorophyll c-containing algae. In: Govindjee, Amesz J, Fork DC (eds) Light emission by plants and bacteria. Academic Press, Massachusetts, pp 497–537
Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, Boston, pp 1–42
Hu Q, Miyashita H, Iwasaki I, Kurano N, Miyachi S, Iwaki M, Itoh S (1998) A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis. Proc Natl Acad Sci USA 95:13319–13323
Li Y, Scales N, Blankenship RE, Willows RD, Chen M (2012) Extinction coefficient for red-shifted chlorophylls: chlorophyll d and chlorophyll f. Biochim Biophys Acta 1817:1292–1298
Li Y, Cai ZL, Chen M (2013) Spectroscopic properties of chlorophyll f. J Phys Chem B 117:11309–11317
Mimuro M (2004) Photon capture, exciton migration and trapping and fluorescence emission in cyanobacteria and red algae. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, Boston, pp 173–195
Mimuro M, Akimoto S, Yamazaki I, Miyashita H, Miyachi S (1999) Fluorescence properties of chlorophyll d-dominating prokaryotic alga, Acaryochloris marina: studies using time-resolved fluorescence spectroscopy on intact cells. Biochim Biophys Acta 1412:37–46
Mimuro M, Hirayama K, Uezono K, Miyashita H, Miyachi S (2000) Uphill energy transfer in a chlorophyll d-dominating oxygenic photosynthetic prokaryote, Acaryochloris marina. Biochim Biophys Acta 1456:27–34
Mimuro M, Murakami A, Tomo T, Tsuchiya T, Watabe K, Yokono M, Akimoto S (2011) Molecular environments of divinyl chlorophylls in Prochlorococcus and Synechocystis: differences in fluorescence properties with chlorophyll replacement. Biochim Biophys Acta 1807:471–481
Murata N, Satoh K (1986) Absorption and fluorescence emission by intact cells, chloroplasts, and chlorophyll-protein complexes. In: Govindjee, Amesz J, Fork DC (eds) Light emission by plants and bacteria. Academic Press, Massachusetts, pp 137–159
Nakayama K, Yamaoka T, Katoh S (1979) Chromatographic separation of photosystems I and II from the thylakoid membrane isolated from a thermophilic blue-green alga. Plant Cell Physiol 20:1565–1576
Nakayama K, Mimuro M, Nishimura Y, Yamazaki I, Okada M (1994) Kinetic analysis of energy transfer processes in LHC II isolated from a siphonous green alga Bryopsis maxima with use of a picosecond fluorescence spectroscopy. Biochim Biophys Acta 1188:117–124
Papagiannakis E, van Stokkum IHM, Fey H, Büchel C, van Grondelle R (2005) Spectroscopic characterization of the excitation energy transfer in the fucoxanthin–chlorophyll protein of diatoms. Photosynth Res 86:241–250
Rüdiger W, Schoch S (1988) Chlorophylls. In: Goodwin TW (ed) Plant pigments. Academic Press, Massachusetts, pp 1–59
Schenderlein M, Çetin M, Barber J, Telfer A, Schlodder E (2008) Spectroscopic studies of the chlorophyll d containing photosystem I from the cyanobacterium, Acaryochloris marina. Biochim Biophys Acta 1777:1400–1408
Schlodder E, Cetin M, Byrdin M, Terekhova IV, Karapetyan NV (2005) P700+- and 3P700-induced quenching of the fluorescence at 760 nm in trimeric photosystem I complexes from the cyanobacterium Arthrospira platensis. Biochim Biophys Acta 1706:53–67
Shubin VV, Murthy SDS, Karapetyan NV, Mohanty P (1991) Origin of the 77 K variable fluorescence at 758 nm in the cyanobacterium Spirulina platensis. Biochim Biophys Acta 1060:28–36
Shubin VV, Bezsmertnaya IN, Karapetyan NV (1992) Isolation from Spirulina membranes of two photosystem I-type complexes one of which contains chlorophyll responsible for the 77 K fluorescence band at 760 nm. FEBS Lett 309:340–342
Tomo T, Okubo T, Akimoto S, Yokono M, Miyashita H, Tsuchiya T, Noguchi T, Mimuro M (2007) Identification of the special pair of photosystem II in a chlorophyll d-dominated cyanobacterium. Proc Natl Acad Sci USA 104:7283–7288
Tomo T, Kato Y, Suzuki T, Akimoto S, Okubo T, Noguchi T, Hasegawa K, Tsuchiya T, Tanaka K, Fukuya M, Dohmae N, Watanabe T, Mimuro M (2008) Characterization of highly purified photosystem I complexes from the chlorophyll d-dominated cyanobacterium Acaryochloris marina MBIC 11017. J Biol Chem 283:18198–18209
Tomo T, Shinoda T, Chen M, Allakhverdiev SI, Akimoto S (2014) Energy transfer processes in chlorophyll f-containing cyanobacteria using time-resolved fluorescence spectroscopy on intact cells. Biochim Biophys Acta 1837:1484–1489
van Grondelle R, Gobets B (2004) Transfer and trapping of excitations in plant photosystems. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, Boston, pp 107–132
van Grondelle R, Dekker JP, Gillbro T, Sundström V (1994) Energy transfer and trapping in photosynthesis. Biochim Biophys Acta 1187:1–65
Willows RD, Li Y, Scheer H, Chen M (2013) Structure of chlorophyll f. Org Lett 15:1588–1590
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education of Japan (23370013 to SA, 22370017 to SA and TT, and 24370025, 26220801 to TT), a grant from JST PRESTO (TT), and a Grant from the Australian Research Council’s Discovery Projects funding scheme (project number DP12101360 to TT). MC holds an Australian Research Council Future Fellowship (FT120100464), and MC thanks the Australian Research Council for support (DP120100286, CE140100015). SIA was supported by Grants from the Russian Science Foundation (No: 14-14-00039).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akimoto, S., Shinoda, T., Chen, M. et al. Energy transfer in the chlorophyll f-containing cyanobacterium, Halomicronema hongdechloris, analyzed by time-resolved fluorescence spectroscopies. Photosynth Res 125, 115–122 (2015). https://doi.org/10.1007/s11120-015-0091-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0091-3