Abstract
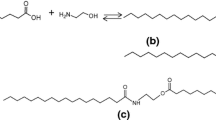
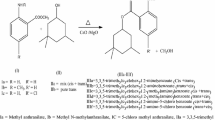
For the first time, in this work, C18 fatty acid amidation with equimolar amounts of ethanolamine was studied both in the absence and presence of heterogeneous catalysts in hexane as a solvent at 180 °C. The products, saturated, and especially unsaturated fatty alkanol amides, which exhibit endocannabinoid activities, have been conventionally prepared using toxic and corrosive homogeneous catalysts. The results revealed that noncatalytic thermal amidation of stearic acid proceeding much faster for stearic than for oleic or linoleic acids. Furthermore, microporous H-Beta-150 was the most active catalyst. Mesoporous, H-MCM-41 with lower acidity was also active in oleic acid amidation, whereas more acidic, mesoporous H-MCM-36 gave lower yield due to formation of esteramides.







Similar content being viewed by others
References
Avraham Y, Katzhendler J, Vorobiev L, Merchavia S, Listman C, Kunkes E, Harfoush F, Salameh S, Ezra AF, Grogoriadis NC, Berry EM, Najajreh Y (2013) Novel acylethanolamide derivatives that modulate body weight through enhancement of hypothalamic pro-opiomelanocortin (POMC) and/or decreased neuropeptide Y (NPY). J Med Chem 56:1811–1829
Tkacheva A, Dosmagambetova I, Chapelliere Y, Mäki-Arvela P, Hachemi I, Savela R, Leino R, Viegas C, Kumar N, Eränen K, Hemming J, Smeds A, Murzin DYu (2015) Pharmaceuticals and surfactants from alga-derived feedstock: amidation of fatty acids and their derivatives. ChemSusChem 8:2670–2680
Sheldon RA (2009) The E factor: fifteen years on. Green Chem 9:1273–1283
Mäki-Arvela P, Holmbom B, Salmi T, Murzin DYu (2007) Recent progress in synthesis of fine and specialty chemicals from wood and other biomass by heterogeneous catalytic processes. Catal Rev 49(3):197–340
Dhellot JR, Matouba E, Maloumbi MG, Nzikou JM, Safou Ngoma DG, Linder M, Desobry S, Parmentier M (2006) Extraction, chemical composition and nutritional characterization of vegetable oils: case of Amaranthus hybridus (Var 1 and 2) of Congo Brazzaville. Afr J Biotechnol 5(11):1095–1101
Kuehl FA, Jacobs TA, Ganley OH, Ormond RE, Meisinger MAO (1957) The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J Am Chem Soc 70:5577–5578
Wyffels L, DeBruyne S, Blanckkaert P, Lambert DM, DeVos F (2009) Radiosynthesis, in vitro and in vivo evaluation of I-123-labeled ananamide analogous for mapping brain FAAH. Bioorg Med Chem 17:49–56
Wyffels L, Muccioli GG, De Bruyne S, Moerman L, Sambre J, Lambert DM, DeVos F (2009) Synthesis, in vitro and in vivo evaluation, and radiolabeling of aryl ananamide analogues as candidate radioligands for in vivo imaging of fatty acid amide hydrolase in the brain. J Medic Chem 52:4613–4622
Maccarrone M, Vanderstelt M, Rossi A, Veldink GA, Vliegenthart JA, Agro AF (1998) Anandamide hydrolysis by human cells in culture and brain*. J Biol Chem 273:32332–32339
Berdyshev EV (1999) Inhibition of sea urchin fertilization by fatty acid ethanolamides and cannabinoids. Comp Biochem Physiol C 122:327–330
Carnovali M, Ottria R, Pasqualetti S, Banfi G, Ciuffreda P, Mariotti M (2016) Effects of bioactive fatty acid amide derivatives in zebrafish scale model of bone metabolism and disease. Pharmacol Res 104:1–8
Ottria R, Casati S, Ciuffreda P (2012) Optimized synthesis and characterization of N-acylethanolamines and O-acylethanolamines, important family of lipid-signalling molecules. Chem Phys Lipids 165:705–711
Cravatt BF, Prosperogarcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA (1995) Chemical characterization of a family of brain lipids that induce sleep. Science 268:1506–1509
Mäki-Arvela P, Tkacheva A, Dosmagambetova I, Chapelliere Y, Hachemi I, Kumar N, Aho A, Murzin DYu (2016) Thermal and catalytic amidation of stearic acid with ethanolamine for production of pharmaceuticals and surfactants. Catal Today 59:1151–1164
Musteata M, Musteata V, Dinu A, Florea M, Hoang VT, Trong-On D, Kaliaguine S, Parvulescu VI (2007) Acylation of different amino derivatives with fatty acids on UL-MFI-type catalysts. Pure Appl Chem 79:2059–2068
Wang X, Wang T, Wang X (2012) An Improved method for synthesis of N-stearoyl and N-palmitoylethanolamine. J Am Oil Chem Soc 89:1305–1313
Wang X, Han Z, Chen Y, Jin Q, Huang J, Wang X (2016) Scalable synthesis of oleoyl ethanolamide by chemical amidation in a mixed solvent. J Am Oil Chem Soc 93:125–131
Lundberg H, Tinnis F, Selander N, Adolfsson H (2014) Catalytic amide formation from non-activated carboxylic acids and amines. Chem Soc Rev 43:2714–2742
Wang X, Chen Y, Jin Q, Huang J, Wang X (2013) Synthesis of linoleoyl ethanolamide. J Oleo Sci 62:427–433
Käldström M, Kumar N, Heikkilä T, Tiitta M, Salmi T, Murzin DYu (2010) Formation of furfural in catalytic transformation of levoglucosan over mesoporous materials. ChemCatChem 2:539–546
Fernández-Pérez M, Otero C (2001) Enzymatic synthesis of amide surfactants from ethanolamine. Enz Microb Technol 28:527–536
Poojari Y, Clarson SJ (2013) Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles in organic media. Biocatal Agric Biotechnol 2:7–11
Kresge T, Leonovicz ME, Roth WJ, Vartuli JC, (1992) Mesoporous crystalline material. US patent 509,868,4 1992
Dubinin MM (1989) Fundamentals of the theory of adsorption in micropores of carbon adsorbents: characteristics of their adsorption properties and microporous structures. Carbon 27:457–467
Chen SG, Yang RT (1994) Theoretical basis for the potential theory adsorption isotherms. The Dubinin-Radushkevich and Dubinin-Astakhov equations. Langmuir 10:4244–4249
MAUD program (http://www.ing.unitn.it/~maud) Accessed 20 Jun. 2016
Emeis CA (1993) Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J Catal 141:347–354
Hypercube, Inc. HyperChem®Release 7 for Windows®, January 2002
Mischenko KP, Ravdel AA (Eds) (1972) Handbook of physico-chemical parameters. Chemistry, Leningrad (in Russian)
Käldström M, Kumar N, Heikkilä T, Murzin DYu (2011) Pillared H-MCM-36 mesoporous and H-MCM-22 microporous materials for conversion of levoglucosan: influence of varying acidity. Appl Catal A 397:13–21
Guo W, Xiong C, Huang L, Li Q (2001) Synthesis and characterization of composite molecular sieves comprising zeolite Beta with MCM-41 structures. J Mater Chem 11:1886–1890
de Vekki AB, Mozzhukhina TN (1997) Heterogeneous catalytical amidation of m-toluic acid by diethanolamine. Pet Chem 37:326–336
Rich MR (1993) Conformational analysis of arachidonic and related fatty acids using molecular cell research. Biochim et Biophys Acta (BBA)-Mol Cell Res 1178:87–96
Tolvanen P, Mäki-Arvela P, Kumar N, Eränen K, Sjöholm R, Hemming J, Holmbom B, Salmi T, Yu Murzin D (2007) Thermal and catalytic oligomerisation of fatty acids. Appl Catal A 330:1–11
Tolvanen P, Mäki-Arvela P, Eränen K, Wärnå J, Holmbom B, Salmi T, Murzin DY (2008) Thermal polymerisation and autoxidation of technical grade linoleic acid. J Am Oil Chem Soc 85:567–572
Charville H, Jackson D, Hodges G, Whiting A (2014) The thermal and boron-catalysed direct amide formation reactions: mechanistically understudied yet important processes. Chem Commun 46:1813–1823
Lilja J, Murzin DYu, Salmi T, Aumo J, Mäki-Arvela P, Sundell M (2002) Esterification of different acids over heterogeneous and homogeneous catalyst and correlation with the Taft equation. J Mol Catal A 182–183:555–563
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mäki-Arvela, P., Kumar, N., Chapelliere, Y. et al. Kinetics in the thermal and catalytic amidation of C18 fatty acids with ethanolamine for the production of pharmaceuticals. Reac Kinet Mech Cat 120, 15–29 (2017). https://doi.org/10.1007/s11144-016-1086-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1086-6