Abstract
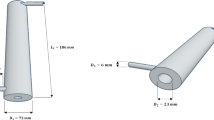
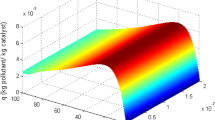
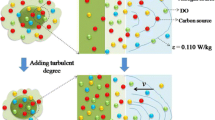
In this study, we performed a computational fluid dynamics (CFD) modeling of tetracycline photocatalytic degradation using rGO/ZnO/Cu as the photocatalyst. The photoreactor was an unbaffled dished bottom tank with a backswept impeller and four lamp tubes. The CFD equations were solved for light intensity, velocity, pressure, and concentration fields in time-dependent modes of the fluid regime. Our model was more complex than the previous studies in several ways: we modeled the system is 3D instead of 2D, allowed variation of light intensity and pressure throughout the photoreactor, and traced the change of key parameters over time during the initiation phase until the steady state was reached. The flow pattern was solved and visualized in the presence of mixer in vertical and horizontal cuts. Experimentally measured concentrations over time matched model predictions well but also indicated the need for yet more complex models incorporating more factors that affect reaction rates.









Similar content being viewed by others
References
Kumaresan T, Joshi JB (2006) Effect of impeller design on the flow pattern and mixing in stirred tanks. Chem Eng J 115:173–193. https://doi.org/10.1016/j.cej.2005.10.002
Lamberto DJ, Alvarez MM, Muzzio FJ (1999) Experimental and computational investigation of the laminar flow structure in a stirred tank. Chem Eng Sci 54:919–942. https://doi.org/10.1016/S0009-2509(98)00275-9
Hartmann H, Derksen JJ, van den Akker HEA (2006) Mixing times in a turbulent stirred tank by means of LES. AIChE J 52:3696–3706. https://doi.org/10.1002/aic.10997
Aubin J, Fletcher DF, Xuereb C (2004) Modeling turbulent flow in stirred tanks with CFD: the influence of the modeling approach, turbulence model and numerical scheme. Exp Therm Fluid Sci 28:431–445. https://doi.org/10.1016/j.expthermflusci.2003.04.001
Huang W, Li K (2013) CFD simulation of flows in stirred tank reactors through prediction of momentum source. In: Nuclear reactor thermal hydraulics and other applications. InTech, p 13
Buwa V, Dewan A, Nassar AF, Durst F (2006) Fluid dynamics and mixing of single-phase flow in a stirred vessel with a grid disc impeller: experimental and numerical investigations. Chem Eng Sci 61:2815–2822. https://doi.org/10.1016/j.ces.2005.10.066
Malayeri M, Lee CS, Haghighat F, Klimes L (2020) Modeling of gas-phase heterogeneous photocatalytic oxidation reactor in the presence of mass transfer limitation and axial dispersion. Chem Eng J 386:124013. https://doi.org/10.1016/j.cej.2020.124013
Cai Y, Liu L, Tian H et al (2019) Adsorption and desorption performance and mechanism of tetracycline hydrochloride by activated carbon-based adsorbents derived from sugar cane bagasse activated with ZnCl2. Molecules 24:4534. https://doi.org/10.3390/molecules24244534
Jing X, Wang Y, Liu W et al (2014) Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem Eng J 248:168–174. https://doi.org/10.1016/j.cej.2014.03.006
Fu Y, Peng L, Zeng Q, Yang Y, Song H, Shao J, Liu S, Gu J (2015) high efficient removal of tetracycline from solution by degradation and flocculation with nanoscale zerovalent iron. Chem Eng J. https://doi.org/10.1016/j.cej.2015.02.070
Ahmadi M, Ramezani H, Jaafarzadeh N (2017) Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J Enviromental Manag 186:55–63. https://doi.org/10.1016/j.jenvman.2016.09.088
Asgharian M, Mehdipourghazi M, Khoshandam B, Keramati N (2020) Experimental design and RSM modeling of tetracycline photocatalytic degradation using rGO/ZnO/Cu. Desalin Water Treat 195:177–185. https://doi.org/10.5004/dwt.2020.25878
Dehghan A, Dehghani MH, Nabizadeh R et al (2018) Adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride from aqueous solutions using 3D hierarchical mesoporous BiOI: synthesis and characterization, process optimization, adsorption and degradation modeling. Chem Eng Res Des 129:217–230. https://doi.org/10.1016/j.cherd.2017.11.003
Gao B, Chen W, Liu J et al (2018) Continuous removal of tetracycline in a photocatalytic membrane reactor (PMR) with ZnIn2S4 as adsorption and photocatalytic coating layer on PVDF membrane. J Photochem Photobiol A Chem 364:732–739. https://doi.org/10.1016/j.jphotochem.2018.07.008
Khan MH, Bae H, Jung J (2010) Tetracycline degradation by ozonation in the aqueous phase: proposed degradation intermediates and pathway. J Hazard Mater 181:659–665. https://doi.org/10.1016/j.jhazmat.2010.05.063
Safari GH, Hoseini M, Seyedsalehi M et al (2015) Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int J Environ Sci Technol 12:603–616. https://doi.org/10.1007/s13762-014-0706-9
Tran Thi VH, Lee BK (2017) Great improvement on tetracycline removal using ZnO rod-activated carbon fiber composite prepared with a facile microwave method. J Hazard Mater 324:329–339. https://doi.org/10.1016/j.jhazmat.2016.10.066
Ao X, Sun W, Li S et al (2019) Degradation of tetracycline by medium pressure UV-activated peroxymonosulfate process: influencing factors, degradation pathways, and toxicity evaluation. Chem Eng J 361:1053–1062. https://doi.org/10.1016/j.cej.2018.12.133
Wang H, Yao H, Pei J et al (2015) Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO. Desalin Water Treat. https://doi.org/10.1080/19443994.2015.1103309
Wang H, Yao H, Pei J et al (2016) Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO. Desalin Water Treat 57:19981–19987. https://doi.org/10.1080/19443994.2015.1103309
Mondaca A, Giraldo A, Pen G et al (2009) Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal Today 144:100–105. https://doi.org/10.1016/j.cattod.2008.12.031
Liu H, Yang Y, Kang J et al (2012) Removal of tetracycline from water by Fe-Mn binary oxide. J Environ Sci 24:242–247. https://doi.org/10.1016/S1001-0742(11)60763-8
Drogui RDP (2013) Tetracycline antibiotics in the environment: a review. Environ Chem Lett 11:209–227. https://doi.org/10.1007/s10311-013-0404-8
Ván R, Kristina C (2015) ZnO/graphite composites and its antibacterial activity at different conditions. J Photochem Photobiol B Biol 151:256–263. https://doi.org/10.1016/j.jphotobiol.2015.08.017
Mohammad A, Ahmad K, Qureshi A et al (2018) Zinc oxide-graphitic carbon nitride nanohybrid as an efficient electrochemical sensor and photocatalyst. Sensors Actuators B Chem. https://doi.org/10.1016/j.snb.2018.07.086
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res 88:428–448. https://doi.org/10.1016/j.watres.2015.09.045
Janotti A, Van de Walle CG (2009) Fundamentals of zinc oxide as a semiconductor. Reports Prog Phys 72:126501. https://doi.org/10.1088/0034-4885/72/12/126501
Das AK, Misra P, Ajimsha RS et al (2017) Electron interference effects and strong localization in Cu doped ZnO thin films. Mater Sci Semicond Process 68:275–278. https://doi.org/10.1016/j.mssp.2017.06.024
Torres-Hernández JR, Ramírez-Morales E, Rojas-Blanco L et al (2015) Structural, optical and photocatalytic properties of ZnO nanoparticles modified with Cu. Mater Sci Semicond Process 37:87–92. https://doi.org/10.1016/j.mssp.2015.02.009
Putri LK, Ong W-J, Chang WS, Chai S-P (2015) Heteroatom doped graphene in photocatalysis: a review. Appl Surf Sci 358:2–14. https://doi.org/10.1016/j.apsusc.2015.08.177
Guinea F, Katsnelson MI, Geim AK (2010) Energy gaps and a zero-field quantum hall effect in graphene by strain engineering. Nat Phys 6:30–33. https://doi.org/10.1038/nphys1420
Kang W, Jimeng X, Xitao W (2016) The effects of ZnO morphology on photocatalytic efficiency of ZnO/RGO nanocomposites. Appl Surf Sci 360:270–275. https://doi.org/10.1016/j.apsusc.2015.10.190
Pant HR, Park CH, Pokharel P et al (2013) ZnO micro-flowers assembled on reduced graphene sheets with high photocatalytic activity for removal of pollutants. Powder Technol 235:853–858. https://doi.org/10.1016/j.powtec.2012.11.050
Ravichandran K, Chidhambaram N, Gobalakrishnan S (2016) Copper and Graphene activated ZnO nanopowders for enhanced photocatalytic and antibacterial activities. J Phys Chem Solids 93:82–90. https://doi.org/10.1016/j.jpcs.2016.02.013
Asgharian M, Mehdipourghazi M, Khoshandam B, Keramati N (2019) Photocatalytic degradation of methylene blue with synthesized rGO/ZnO/Cu. Chem Phys Lett. https://doi.org/10.1016/j.cplett.2019.01.037
Casado C, Marugán J, Timmers R et al (2017) Comprehensive multiphysics modeling of photocatalytic processes by computational fluid dynamics based on intrinsic kinetic parameters determined in a differential photoreactor. Chem Eng J 310:368–380. https://doi.org/10.1016/j.cej.2016.07.081
Ramos-Huerta LA, Valadés-Pelayo PJ, Llanos AG et al (2021) Development of a new methodology to determine suspended photocatalyst optical properties. Chem Eng J. https://doi.org/10.1016/j.cej.2020.127458
Pakzad L, Ein-Mozaffari F, Upreti SR, Lohi A (2013) Evaluation of the mixing of non-newtonian biopolymer solutions in the reactors equipped with the coaxial mixers through tomography and CFD. Chem Eng J 215–216:279–296. https://doi.org/10.1016/j.cej.2012.10.060
Foukrach M, Bouzit M, Ameur H, Kamla Y (2020) Effect of agitator’s types on the hydrodynamic flow in an agitated tank. Chinese J Mech Eng. https://doi.org/10.1186/s10033-020-00454-2
Boyjoo Y, Ang M, Pareek V (2013) Light intensity distribution in multi-lamp photocatalytic reactors. Chem Eng Sci 93:11–21. https://doi.org/10.1016/j.ces.2012.12.045
Zhu XD, Wang YJ, Sun RJ, Zhou DM (2013) Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 92:925–932. https://doi.org/10.1016/j.chemosphere.2013.02.066
Lv C, Lan X, Wang L et al (2019) Rapidly and highly efficient degradation of tetracycline hydrochloride in wastewater by 3D IO-TiO2-CdS nanocomposite under visible light. Environ Technol. https://doi.org/10.1080/09593330.2019.1629183
Mahbubul IM (2019) Optical properties of nanofluid
Ahmad SHA, Saidur R, Mahbubul IM, Al-Sulaiman FA (2017) Optical properties of various nanofluids used in solar collector: a review. Renew Sustain Energy Rev 73:1014–1030. https://doi.org/10.1016/j.rser.2017.01.173
Hoseini SS, Najafi G, Ghobadian B, Akbarzadeh AH (2020) Impeller shape-optimization of stirred-tank reactor: CFD and fluid structure interaction analyses. Chem Eng J. https://doi.org/10.1016/j.cej.2020.127497
Ramírez-Cruz J, Salinas-Vázquez M, Ascanio G et al (2020) Mixing dynamics in an uncovered unbaffled stirred tank using large-eddy simulations and a passive scalar transport equation. Chem Eng Sci. https://doi.org/10.1016/j.ces.2020.115658
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asgharian, M., Khoshandam, B., Mehdipourghazi, M. et al. Photocatalytic degradation of tetracycline in a stirred tank: computational fluid dynamic modeling and data validation. Reac Kinet Mech Cat 134, 553–568 (2021). https://doi.org/10.1007/s11144-021-02062-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02062-0