Abstract
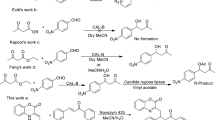
Substituted phenacyl chlorides (1a–f) were cyclocondensed with nucleophiles thiourea (2a) and thiobenzamide (2b) in presence of baker’s yeast (Saccharomyces cerevisiae) as whole-cell enzyme source in acetonitrile at room temperature to obtain 4-(4-substituted phenyl)thiazol-2-amines (3a–f) and 4-(substituted phenyl)-2-phenylthiazoles (4a–f), respectively. Moreover, substituted phenacyl chlorides also reacted with nucleophiles 2-amino-1,3,4-thiadiazole (2c), o-phenylenediamine (2d), 1-amino-2-mercapto-5-phenyl triazole (2e), and pyridin-2-amine (2f) at room temperature in presence of baker’s yeast to give fused heterocycles 6-(4-substituted phenyl)-2-phenylimidazo[2,1-b][1,3,4]thiadiazoles (5a–f), 2-(4-substituted phenyl)quinoxalines (6a–f), 6-(4-substituted phenyl)-3-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines (7a–f), and 2-(4-substituted phenyl)H-imidazo[1,2-a]pyridines (8a–f), respectively. The experimental conditions for these cyclocondensations were optimized to obtain the biodynamic heterocycles in high yield. The unique features of this work are use of baker’s yeast as a cheap and readily available natural source of biocatalyst, noticeable rate acceleration, convenient route to products, cost effectiveness, and scalability.





Similar content being viewed by others
References
E. Vitaku, D.T. Smith, J.T. Njardarson, J. Med. Chem. 57, 10257 (2014)
T. Shiro, T. Fukaya, M. Tobe, Eur. J. Med. Chem. 97, 397 (2015)
R.E. Buntrock, J. Chem. Educ. 89, 1349 (2012)
S. Rasheed, D. Nageswar Rao, P. Das, J. Org. Chem. 80, 9321 (2015)
P. Majumdar, A. Pati, M. Patra, R.K. Behera, A.K. Behera, Chem. Rev. 114, 2942 (2014)
Y.D. Gong, T. Lee, J. Comb. Chem. 12, 393 (2010)
C. Gil, S.B. Se, J. Comb. Chem. 11, 175 (2009)
A.F.M. Fahmy, ARKIVOC 7, 395 (2006)
R. Romagnoli, P.G. Baraldi, F. Prencipe, J. Balzarini, S. Liekens, F. Est evez, Eur. J. Med. Chem. 101, 185 (2015)
Z. Zhuang, M.P. Kung, A. Wilson, C.W. Lee, K. Plossl, C. Hou, D.M. Holtzman, H.F. Kung, J. Med. Chem. 46, 237 (2003)
Y. Jin, M. C. Rho, K. Gajulapati, H.Y. Jung, S. K. Boovanahalli, J. H. Lee, G. Y. Song, J. H. Choi, Y. K. Kim, K. Lee, Y. Choi, Bull. Korean Chem. Soc. 1297 (2009)
B.E.D. Montano, L.C.G. Caro, M.S. Sanchez, A. Monge, E.H. Baltazar, G. Rivera, O.T. Angeles, Bioorg. Med. Chem. 21, 4550 (2013)
Kui Cheng, Jia-Yu. Xue, Hai-Liang Zhu, Bioorg. Med. Chem. Lett. 23, 4235 (2013)
V. Sumangal, B. Poojary, N. Chidananda, T. Arulmoli, S. Shenoy, Eur. J. Med. Chem. 54, 59 (2012)
A. Shaabani, A. Maleki, H. Mofakham, Mol. Divers. 13, 63 (2009)
A. Maleki, Z. Alrezvani, S. Maleki, Catal. Commun. 69, 29 (2015)
A. Maleki, M. Aghaei, N. Ghamari, Chem. Lett. 44, 261 (2015)
A. Maleki, RSC Adv. 4, 64169 (2014)
A. Maleki, H. Movahed, P. Ravaghi, Carbohydr. Polym. 156, 259 (2017)
A. Maleki, M. Aghaei, Ultra Sonochem (2016). doi:10.1016/j.ultsonch.2016.08.024
A. Maleki, Tetrahedron 68, 7827 (2012)
A. Maleki, Tetrahedron Lett. 54, 2055 (2013)
A. Maleki, RSC Adv. 4, 9416 (2014)
A. Maleki, Helv. Chim. Acta 97, 587 (2014)
A. Maleki, M. Kamalzare, Catal. Commun. 53, 67 (2014)
A. Maleki, R. Paydar, RSC Adv. 5, 33177 (2015)
A. Maleki, H. Movahed, R. Paydar, RSC Adv. 6, 13657 (2016)
W.S. Alwan, R. Karpoormath, M.B. Palkar, H.M. Patel, R.A. Rane, M.S. Shaikh, A. Kajee, K.P. Mlisana, Eur. J. Med. Chem. 95, 514 (2015)
A.W. Erian, S.M. Sherif, H.M. Gaber, Molecules 8, 793 (2003)
E. Deau, C.D. Benard, V. Levacher, T. Besson, Tetrahedron 70, 5532 (2014)
T. Aoyama, S. Murata, I. Arai, N. Araki, T. Takido, Y. Suzukib, M. Kodomari, Tetrahedron 62, 3201 (2006)
J. Hammerle, M. Schnurch, N. Iqbal, M.D. Mihovilovic, P. Stanetty, Tetrahedron 66, 8051 (2010)
M. Kodomari, T. Aoyama, Y. Suzuki, Tetrahedron Lett. 43, 1717 (2002)
J. Noei, A.R. Khosropour, Ultrason. Sonochem. 16, 711 (2009)
K. Aghapoor, F. Mohsenzadeh, A. Shakeri, H. Reza Darabi, M. Ghassemzadeh, B. Neumuller, J. Organomet. Chem. 743, 170 (2013)
C. Zhang, Z. Xu, L. Zhang, N. Jiao, Tetrahedron 68, 5258 (2012)
Y. Suzuki, M. Murofushi, K. Manabe, Tetrahedron 69, 470 (2013)
C.Y. Chen, W.P. Huc, M.C. Liu, P.C. Yan, J.J. Wang, M.I. Chung, Tetrahedron 69, 9735 (2013)
V. Jeena, R.S. Robinson, Tetrahedron Lett. 55, 642 (2014)
G. Mekuskiene, P. Vainilavicius, Chem. Heter. Comp. 43, 919 (2007)
Y. Jin, M.C. Rho, K. Gajulapati, H.Y. Jung, S.K. Boovanahalli, J.H. Lee, G.Y. Song, J.H. Choi, Y.K. Kim, K. Lee, Y. Choi, Bull. Korean Chem. Soc. 30, 1297 (2009)
J.P. Wan, S.F. Gan, J.M. Wu, Y. Pan, Green Chem. 11, 1633 (2009)
A. Jakoblinnert, D. Rother, Green Chem. 16, 3475 (2014)
A.D.C. Parenty, L.V. Smith, A.L. Pickering, D.L. Long, L. Cronin, J. Org. Chem. 69, 5934 (2004)
R.N. Patel, ACS Catal. 1, 1059 (2011)
S.E. Milnera, A.R. Maguire, ARKIVOC 1, 321 (2012)
A. Mane, P. Salokhe, P. More, R. Salunkhe, J. Mol. Cat. B Enzymatic 121, 75 (2015)
N. Nir, M. Bahalul, R. Feingersch, T.K. Ezov, Y. Kashi, A. Fishman, Appl. Microbiol. Biotech. 78, 659 (2008)
F. Hollmann, I.W.C.E. Arendsa, D. Holtmann, Green Chem. 13, 2285 (2011)
N.G. Singh, R. Nongrum, C. Kathing, J.W.S. Rani, R. Nongkhlaw, Green Chem. Lett. Rev. 7, 137 (2014)
L. Forti, S. Di Mauro, M.R. Cramarossa, S. Filippucci, B. Turchetti, P. Buzzini, Molecules 20, 10377 (2015)
A.G. Saimaa, R. Lavekara, A.K. Kumarc, J. Sinhaa, Mol. Cat. B Enzymatic 116, 113 (2015)
U.R. Pratap, D.V. Jawale, R.A. Waghmare, D.L. Lingampalle, R.A. Mane, New J. Chem. 35, 49 (2011)
U.R. Pratap, D.V. Jawale, M.R. Bhosle, R.A. Mane, Tetrahedron Lett. 52, 1689 (2011)
U.R. Pratap, D.V. Jawale, B.S. Londhe, R.A. Mane, J. Mol. Cat. B Enzymatic 68, 94 (2011)
A.J. Stasyuk, M. Banasiewicz, M.K. Cyrański, Daniel T. Gryko, J. Org. Chem. 77, 5552 (2012)
V.M. Bangade, B.C. Reddy, P.B. Thakur, B.M. Babu, H.M. Meshram, Tetrahedron Lett. 54, 4767 (2013)
P.M Bonilla, A Perez Cardena, E Quintero Marmol, JL Arias Tellez, GJ Mena Rejon, Het. Chem. doi:10.1002/hc.250
D. Kumar, N.M. Kumar, G. Patel, S. Gupta, Rajender S. Varma, Tetrahedron Lett. 52, 1983 (2011)
K. Tiwari, P.K. Verma, S.B. Singh, J. Singh, Synth. Commun. 42, 3021 (2012)
K.S. Vadagaonkar, H.P. Kalmode, K. Murugan, A.C. Chaskar, RSC Adv. 5, 5580 (2015)
Z.G. Le, Z.B. Xie, J.P. Xu, Molecules 17, 13368 (2012)
Y.A. Sonawane, S.B. Phadtare, B.N. Borse, A.R. Jagtap, G.S. Shankarling, Org. Lett. 12, 1456 (2010)
A. Kademi, B. Lee, A. Houde, Indian J. Biotechnol. 2, 346 (2003)
M.J. Brumlik, J.T. Buckley, J. Bacteriol. 178, 2060 (1996)
Acknowledgements
The authors are grateful to Professor D. B. Ingle for invaluable discussion and guidance. The authors are very grateful to CDRI, Lucknow for providing spectral facilities. L.D.K. is also grateful to U.G.C., New Delhi, India for financial assistance in the form of a UGC-NET Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khillare, L.D., Pratap, U.R., Bhosle, M.R. et al. Syntheses of biodynamic heterocycles: baker’s yeast-assisted cyclocondensations of organic nucleophiles and phenacyl chlorides. Res Chem Intermed 43, 4327–4337 (2017). https://doi.org/10.1007/s11164-017-2880-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2880-0