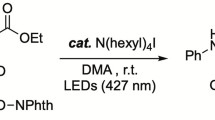
The possibility of photocatalytic C(sp3)–H activation of aliphatic amines with the participation of decatungstate anion and their subsequent interaction with an acrylic acid ester has been demonstrated. Irradiation of reaction mixtures containing an aliphatic amine in the form of trifluoroacetic acid salt, acrylic acid tert-butyl ester, and sodium decatungstate Na4W10O32 or tetra-n-butylammonium has been shown to result in the formation of branched-chain amino acid esters.




Similar content being viewed by others
References
S. Roslin and L. R. Odell, Eur. J. Org. Chem., 2017, No. 15, 1993-2007 (2017), https://doi.org/10.1002/ejoc.201601479.
H. Yi., G. Zhang, H. Wang, et al., Chem. Rev., 117, No. 13, 9016-9085 (2017), https://doi.org/10.1021/acs.chemrev.6b00620.
N. Hoffman, Synthesis, 48, No. 12, 1782-1802 (2016), https://doi.org/10.1055/s-0035-1561425.
L. Capaldo and D. Ravelli, Eur. J. Org. Chem., 2017, No. 15, 2056-2071 (2017), https://doi.org/10.1002/ejoc.201601485.
D. Ravelli, S. Plotti, and M. Fagnoni, Chem. Rev., 116, No. 17, 9850-9913 (2016).
P. A. Champagne, J. Desroches, J.-D. Hamel, et al., Chem. Rev., 115, No. 17, 9073-9174 (2015), https://doi.org/10.1021/acs.chemrev.5b00662.
S. Bloom, J. L. Knippel, and T. Lectka, Chem. Sci., 5, No. 3, 1175-1178 (2014), https://doi.org/10.1039/C3SC53261E.
S. D. Halperin, H. Fan, S. Chang, et al., Angew. Chem., 126, No. 18, 4778-4781 (2014), https://doi.org/10.1002/anie.201400420.
A. V. Kozytskiy, Ya. V. Panasyuk, and A. M. Mishura, Theor. Exp. Chem., 54, No. 5, 322-330 (2018), https://doi.org/10.1007/s11237-018-9577-3.
I. B. Perry, T. F. Brewer, P. J. Sarver, et al., Nature, 560, No. 7716, 70-75 (2018), https://doi.org/10.1038/s41586-018-0366-x.
T. Fukuyama, K. Yamada, T. Nishikawa, et al., Chem. Lett., 47, No. 2, 207-209 (2018), https://doi.org/10.1246/cl.171068.
T. Fukuyama, T. Nishikawa, K. Yamada, et al., Org. Lett., 19, No. 23, 6436-6439 (2017), https://doi.org/10.1021/acs.orglett.7b03339.
I. N. Lykakis, E. Evgenidou, and M. Orfanopoulos, Curr. Org. Chem., 16, No. 20, 2400-2414 (2012), https://doi.org/10.2174/13852721280350092.
D. Ravelli, S. Plotti, M. Fagnoni, Acc. Chem. Res., 49, No. 10, 2232-2242 (2016), https://doi.org/10.1021/acs.accounts.6b00339.
A. Chemseddine, C. Sanchez, J. Livage, et al., Inorg. Chem., 23, No. 17, 2609-2613 (1984) https://doi.org/10.1021/ic00185a014.
C. Tanielian, Coord. Chem. Rev., 178-180, part 2, 1165-1181 (1998).
K. Suzuki, N. Mazino, and M. Yamaguchi, ACS. Catal., 8, No. 11, 10809-10825 (2018), https://doi.org/10.1021/acscatal.8b03498.
V. D. Waele, O. Poizat, M. Fagnoni, et al., ACS. Catal., 6, No. 10, 7174-7182 (2016), https://doi.org/10.1021/acscatal.6b01984.
I. Texier., J. A. Delaire, and C. Giannotti, Phys. Chem. Chem. Phys., 2, No. 6, 1205-1212 (2000), https://doi.org/10.1039/A908588B.
P. Sykes, Mechanisms in Organic Chemistry [in Russian], Khimiya, Moscow (1991).
Yu. M. Kiselev, Chemistry of Coordination Compounds, Textbook and Practical Training for Universities [in Russian], Yurait, Moscow (2021).
H. M. L. Davies and D. Morton, J. Org. Chem., 81, No. 1, 343-350 (2016), https://doi.org/10.1021/acs.joc.5b02818.
J. Das, S. Guin, and D. Maiti, Chem. Sci., 11, No. 40, 10887-10909 (2020), https://doi.org/10.1039/D0SC04676K.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 57, No. 6, pp. 370-374, November-December, 2021.
Rights and permissions
About this article
Cite this article
Kozytskiy, A.V., Bielousov, O.P. Photocatalytic C(sp3)–H Activation of Aliphatic Amines by Using Decatungstate Anion to Obtain Aminoacids. Theor Exp Chem 57, 437–442 (2022). https://doi.org/10.1007/s11237-022-09713-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-022-09713-w