Abstract
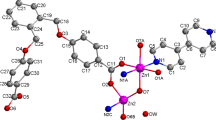
A new one-dimensional cyanide-based coordination polymer comprising Sm(III) and octacyanomolybdate(V) building blocks was prepared and characterized through single-crystal X-ray diffraction and elemental analyses. The new compound consisted of one-dimensional chains of [Sm(terpy)(DMF)4] [Mo(CN)8] (complex 1) (terpy: 2,2′:6′,2′′-terpyridine; DMF: N,N-dimethylformamide), where each Mo(CN)8 entity served as a bis-monodentate bridging ligand of two Sm(III) ions alternately through two of its six cyanide groups at cis positions. The corresponding chains were formed by hydrogen bonds, and van der Waals interactions to form a two-dimensional supramolecular structure that stabilized the entire molecule. Considering all the intermolecular interactions during the simplification procedure, we obtained a description of the molecular packing. The calculation results indicated that the underlying net corresponds to a new topology with the following point symbol {337.468.530.6}{38.42}. Density functional theory studies were also performed to elucidate the electronic structure within the entire complex. The good luminescence of the complex makes it a suitable material for construction of photoluminescent materials, to explore the intrinsic optical properties of lanthanides, and in sensing for detection of different hazardous/explosive materials.







Similar content being viewed by others
References
Chelebaeva E et al (2008) A luminescent and magnetic cyano-bridged Tb3+−Mo5+ coordination polymer: toward multifunctional materials. Inorg Chem 47:775–777. https://doi.org/10.1021/ic702192k
Muddassir M (2020) Syntheses, structural characterization, and thermal behavior of cyanide-bridged [2 + 2]-type tetranuclear rectangle-based molecule constructed from Tm(III) and hexacyanocobaltate(III). Trans Met Chem 45:317–323. https://doi.org/10.1007/s11243-020-00382-z
Muddassir M, Alarifi A, Afzal M, Sepay N (2020) Newly designed Mn(III)–W(V) bimetallic assembly built by manganese (III) Schiff–base and octacyanotungstate(V) building blocks: structural topologies, and magnetic features. Appl Organomet Chem n/a:e5914. https://doi.org/10.1002/aoc.5914
Tong Y-Z et al (2013) Nine cyanide-bridged bimetallic magnetic chains derived from octacyanomolybdate(v) and lanthanide(iii) building blocks. CrystEngComm 15:9906–9915. https://doi.org/10.1039/C3CE41048J
Chelebaeva E et al (2009) Luminescent and magnetic cyano-bridged coordination polymers containing 4d–4f ions: toward multifunctional materials. Inorg Chem 48:5983–5995. https://doi.org/10.1021/ic900378d
Rodriguez A et al (2005) Hexacyanocobaltate(III) anions as precursors of Co(II)–Ni(II) cyano-bridged multidimensional assemblies: hydrothermal syntheses, crystal and powder X-ray structures, and magnetic properties. Inorg Chem 44:8399–8406. https://doi.org/10.1021/ic0511672
Tanase S, Ferbinteanu M, Cimpoesu F (2011) Rationalization of the lanthanide-ion-driven magnetic properties in a series of 4f–5d cyano-bridged chains. Inorg Chem 50:9678–9687. https://doi.org/10.1021/ic201427w
Chorazy S, Wyczesany M, Sieklucka B (2017) Lanthanide photoluminescence in heterometallic polycyanidometallate-based coordination networks. Molecules 22:1902. https://doi.org/10.3390/molecules22111902
Long J, Chamoreau L-M, Mathonière C, Marvaud V (2009) Photoswitchable heterotrimetallic chain based on octacyanomolybdate, copper, and nickel: synthesis, characterization, and photomagnetic properties. Inorg Chem 48:22–24. https://doi.org/10.1021/ic801901j
Muddassir M (2020) A new 1D Cu(II)-W(CN)8 based coordination polymer: crystallographic structural architecture, Hirshfeld surface, DFT and luminescent analyses. J Organomet Chem. https://doi.org/10.1016/j.jorganchem.2020.121499
Qian S-Y, Zhou H, Yuan A-H, Song Y (2011) Syntheses, structures, and magnetic properties of five novel octacyanometallate-based lanthanide complexes with helical chains. Cryst Growth Des 11:5676–5681. https://doi.org/10.1021/cg201217a
Gao Y et al (2018) Cyanide-bridged coordination polymers constructed from lanthanide ions and octacyanometallate building-blocks. Inorg Chem Front 5:1967–1977. https://doi.org/10.1039/C8QI00357B
Chorazy S, Rams M, Nakabayashi K, Sieklucka B, Ohkoshi S-I (2016) White light emissive DyIII single-molecule magnets sensitized by diamagnetic [CoIII(CN)6]3- linkers. Chem A Eur J 22:7371–7375. https://doi.org/10.1002/chem.201601244
Prins F et al (2007) Long-range magnetic ordering in a TbIII–MoV cyanido-bridged quasi-one-dimensional complex. Angew Chem Int Ed 46:6081–6084. https://doi.org/10.1002/anie.200701847
Wang Z-X et al (2006) A sodalite-like framework based on octacyanomolybdate and neodymium with guest methanol molecules and neodymium octahydrate ions. Angew Chem Int Ed 45:3287–3291. https://doi.org/10.1002/anie.200600455
Ishikawa N, Sugita M, Ishikawa T, Koshihara S-Y, Kaizu Y (2003) Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J Am Chem Soc 125:8694–8695. https://doi.org/10.1021/ja029629n
Andruh M, Costes J-P, Diaz C, Gao S (2009) 3d–4f combined chemistry: synthetic strategies and magnetic properties. Inorg Chem 48:3342–3359. https://doi.org/10.1021/ic801027q
Stoian SA et al (2010) Mössbauer, electron paramagnetic resonance, and magnetic susceptibility studies on members of a new family of cyano-bridged 3d–4f complexes. Demonstration of anisotropic exchange in a Fe–Gd complex. Inorg Chem 49:3387–3401. https://doi.org/10.1021/ic902516r
Alexandrov EV, Virovets AV, Blatov VA, Peresypkina EV (2015) Topological motifs in cyanometallates: from building units to three-periodic frameworks. Chem Rev 115:12286–12319. https://doi.org/10.1021/acs.chemrev.5b00320
Liu Y et al (2020) Cyanometallate-bridged didysprosium single-molecule magnets constructed with single-ion magnet building block. Inorg Chem 59:687–694. https://doi.org/10.1021/acs.inorgchem.9b02948
Arczyński M, Stanek J, Sieklucka B, Dunbar KR, Pinkowicz D (2019) Site-selective photoswitching of two distinct magnetic chromophores in a propeller-like molecule to achieve four different magnetic states. J Am Chem Soc 141:19067–19077. https://doi.org/10.1021/jacs.9b09576
Zhou H et al (2017) A series of lanthanide(III)-bpdo-octacyanotungstate(V) compounds (bpdo = 4,4′-Bipyridine-N, N′-dioxide) involving the structural transformation from ion pair to three-dimensional pillared layer via a two-dimensional layer. Cryst Growth Des 17:6523–6530. https://doi.org/10.1021/acs.cgd.7b01196
Nakabayashi K et al (2014) Cesium cyano-bridged CoII–MV (M = Mo and W) layered frameworks exhibiting high thermal durability and metamagnetism. Cryst Growth Des 14:6093–6100. https://doi.org/10.1021/cg501250p
Cao F et al (2016) Ferromagnetic polarization: the quantum picture of switching on/off single-molecule magnetism. Inorg Chem 55:5914–5923. https://doi.org/10.1021/acs.inorgchem.6b00255
Qian J et al (2014) A three-dimensional hetero-bimetallic coordination polymer with unusual (4,5)-connected topology and ferrimagnetic property based on octacyanotungstate and polydentate ligand. Cryst Growth Des 14:2288–2295. https://doi.org/10.1021/cg4019053
Podgajny R et al (2003) Coordination polymers based on octacyanometalates(iv{,}v) (M = Mo{,} W) and aliphatic polyamine copper(ii) tectons with [N3] donor atom sets. Dalton Trans. https://doi.org/10.1039/b306422k
Sieklucka B, Podgajny R, Przychodzeń P, Korzeniak T (2005) Engineering of octacyanometalate-based coordination networks towards functionality. Coord Chem Rev 249:2203–2221. https://doi.org/10.1016/j.ccr.2005.02.024
Nowicka B et al (2012) The impact of ligands upon topology and functionality of octacyanidometallate-based assemblies. Coord Chem Rev 256:1946–1971. https://doi.org/10.1016/j.ccr.2012.04.008
Przychodzeń P, Korzeniak T, Podgajny R, Sieklucka B (2006) Supramolecular coordination networks based on octacyanometalates: from structure to function. Coord Chem Rev 250:2234–2260. https://doi.org/10.1016/j.ccr.2006.01.026
Corpinot MK, Bučar D-K (2019) A practical guide to the design of molecular crystals. Cryst Growth Des 19:1426–1453. https://doi.org/10.1021/acs.cgd.8b00972
Hayes R, Warr GG, Atkin R (2015) Structure and nanostructure in ionic liquids. Chem Rev 115:6357–6426. https://doi.org/10.1021/cr500411q
Ma S-L, Ren S, Ma Y, Liao D-Z (2009) Sheet-like of MoV-SmIII assembly containing [MoV(CN)8]3– and Sm3+ ions as building blocks. J Chem Sci 121:421–427. https://doi.org/10.1007/s12039-009-0049-0
Yang X-Z et al (2012) Syntheses, structures and magnetic properties of cyano-bridged Sm(III)M(V) (M = Mo, W) assemblies with zigzag chains. Inorg Chem Commun 24:40–42. https://doi.org/10.1016/j.inoche.2012.07.047
Muddassir M et al (2013) Ion-induced diversity in structure and magnetic properties of hexacyanometalate–lanthanide bimetallic assemblies. CrystEngComm 15:10541–10549. https://doi.org/10.1039/C3CE41704B
Isci H, Roy Mason W (2004) Electronic absorption and MCD spectra for octacyanometallate complexes M(CN)8n−, M = Mo(IV), W(IV), n = 4 and Mo(V), W(V), n = 3. Inorg Chim Acta 357:4065–4072. https://doi.org/10.1016/j.ica.2003.10.024
Chorazy S, Arczynski M, Nakabayashi K, Sieklucka B, Ohkoshi S-I (2015) Visible to near-infrared emission from LnIII(Bis-oxazoline)–[MoV(CN)8] (Ln = Ce–Yb) magnetic coordination polymers showing unusual lanthanide-dependent sliding of cyanido-bridged layers. Inorg Chem 54:4724–4736. https://doi.org/10.1021/acs.inorgchem.5b00040
Chorazy S et al (2014) Multifunctionality in bimetallic LnIII[WV(CN)8]3– (Ln = Gd, Nd) coordination helices: optical activity, luminescence, and magnetic coupling. Chem A Eur J 20:7144–7159. https://doi.org/10.1002/chem.201304772
Carter KP, Pope SJA, Cahill CL (2014) A series of Ln-p-chlorobenzoic acid–terpyridine complexes: lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm 16:1873–1884. https://doi.org/10.1039/C3CE42267D
de Bettencourt-Dias A (2007) Lanthanide-based emitting materials in light-emitting diodes. Dalton Trans. https://doi.org/10.1039/b702341c
Bünzli J-CG (2006) Benefiting from the unique properties of lanthanide ions. Acc Chem Res 39:53–61. https://doi.org/10.1021/ar0400894
Song S, Li X, Zhang Y-H, Huo R, Ma D (2014) White light emission by a lanthanide doped Sm(iii) framework constructed from 4-sulfobenzoate and 1H-imidazo[4,5-f][1,10]-phenanthroline. Dalton Trans 43:5974–5977. https://doi.org/10.1039/C3DT53139B
Singh UP, Goel N, Singh G, Srivastava P (2012) Syntheses, structural and thermal studies of Eu(III) and Gd(III) complexes with 2,6-dinitrophenol and 1,10-phenanthroline/2,2′-bipyridine ligands. Inorg Chim Acta 387:294–307. https://doi.org/10.1016/j.ica.2012.02.009
Ma X, Li X, Cha Y-E, Jin L-P (2012) Highly thermostable one-dimensional lanthanide(III) coordination polymers constructed from benzimidazole-5,6-dicarboxylic acid and 1,10-phenanthroline: synthesis, structure, and tunable white-light emission. Cryst Growth Des 12:5227–5232. https://doi.org/10.1021/cg300932a
Garino C et al (2007) Spectroscopic and computational studies of a Ru(II) terpyridine complex: the importance of weak intermolecular forces to photophysical properties. Inorg Chem 46:8752–8762. https://doi.org/10.1021/ic7010343
Leipoldt JG, Bok LDC, Cilliers PJ (1974) The preparation of potassium octacyanotungstate(IV) dihydrate. Z Anorg Allg Chem 407:350–352. https://doi.org/10.1002/zaac.19744070311
Acknowledgements
The authors extend their sincere appreciation to the Deputyship for Research and Innovation, Ministry of Education, Saudi Arabia for funding this research work through project no. IFKSURG-1440-076. The authors also thank the Deanship of Scientific Research and RSSU at King Saud University for technical support and are grateful for the positive and highly valuable suggestions from the reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muddassir, M., Alarifi, A. & Afzal, M. Synthesis, structural topology, DFT, and photoluminescence properties of Sm(III) and octacyanomolybdate(V) building-block-based 1-D chain complex. Transit Met Chem 46, 129–137 (2021). https://doi.org/10.1007/s11243-020-00429-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00429-1