Abstract
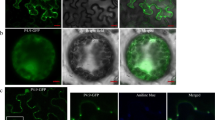
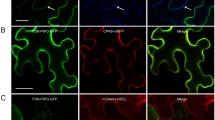
Indian cassava mosaic virus (ICMV), responsible for the cassava mosaic disease in India, harbours two circular genomic components, DNA-A and DNA-B; the former being responsible for the encapsidation and replication and the latter for intra- and inter-cellular movement of the viral DNA. Two proteins, encoded by DNA-B, the movement protein (MP) and the nuclear shuttle protein (NSP), act in concert on the newly replicated viral DNA to move it from the nucleus to the cell periphery. To map the functional domains of NSP, the intra-cellular localization of its full-length protein and deletion derivatives was studied in the epidermal cells of detached leaves of the laboratory host plant, Nicotiana benthamiana, where the target proteins were transiently expressed as GFP fusions. This analysis revealed domains for nuclear localization at the N-terminus, as well as for localization towards the cell periphery both at the C-terminus and center of the NSP.




Similar content being viewed by others
References
Patil BL, Fauquet CM (2009) Cassava mosaic geminiviruses: actual knowledge and perspectives. Mol Plant Pathol 10:685–701
Rothenstein D, Briddon RW, Haible D, Stanley J, Frischmuth T, Jeske H (2005) Biolistic infection of cassava using cloned components of Indian cassava mosaic virus. Arch Virol 150:1669–1675
Rothenstein D, Haible D, Dasgupta I, Dutt N, Patil BL, Jeske H (2005) Biodiversity and recombination of cassava-infecting begomoviruses from southern India. Arch Virol 150(8):1669–1675. https://doi.org/10.17007/s00705-005-0520-2
Patil BL, Rajasubramaniam S, Bagchi C, Dasgupta I (2005) Both Indian cassava mosaic virus and Sri Lankan cassava mosaic virus are found in India and exhibit high variability as assessed by PCR-RFLP. Arch Virol 150(2):389–397. https://doi.org/10.1007/s00705-004-0399-3
Jeske H (2009) Geminiviruses. Curr Top Microbiol Immunol 331:185–226. https://doi.org/10.1007/978-3-540-70972-5_11
Böttcher B, Unseld S, Ceulemans H, Russell RB, Jeske H (2004) Geminate structures of African cassava mosaic virus. J Virol 78:6709–6714
Aberle HJ, Rütz ML, Karayavuz M, Frischmuth S, Wege C, Hülser D, Jeske H (2002) Localizing BC1 movement protein of Abutilon mosaic geminivirus in yeasts by subcellular fractionation and freeze fracture immunolabelling. Arch Virol 147:103–107
Noueiry AO, Lucas WJ, Gilbertson RL (1994) Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 76:925–932
Pascal E, Sanderfoot AA, Ward BM, Medville R, Turgeon R, Lazarowitz SG (1994) The geminivirus BR1 movement protein binds single- stranded DNA and localizes to the cell nucleus. Plant Cell 6:995–1006
Sanderfoot AA, Ingham DJ, Lazarowitz SG (1996) A viral movement protein as a nuclear shuttle. The geminivirus BR1 movement protein contains domains essential for interaction with BL1 and nuclear localization. Plant Physiol 110:23–33
Happle A, Jeske H, Kleinow T (2021) Dynamic subcellular distribution of begomoviral nuclear shuttle and movement proteins. Virology 562:158–175. https://doi.org/10.1016/j.virol.2021.07.014
Sanderfoot AA, Lazarowitz SG (1995) Cooperation in viral movement: the geminivirus BL1 movement protein interacts with BR1 and redirects it from the nucleus to the cell periphery. Plant Cell 7:1185–1194
Ward BM, Lazarowitz SG (1999) Nuclear export in plants. Use of geminivirus movement proteins for a cell-based export assay. Plant Cell 11:1267–1276
Zhang SC, Ghosh R, Jeske H (2001) Subcellular targeting domains of Abutilon mosaic geminivirus movement protein BC1. Arch Virol 147:2349–2363
Zhang SC, Wege C, Jeske H (2001) Movement proteins of Abutilon mosaic geminivirus are cotransported in and between cells of sink but not of the source leaves as detected by green fluorescent protein tagging. Virology 290:249–260
Ward BM, Lazarowitz SG (1999) Nuclear export in plants: use of geminivirus movement proteins for a cell-based export assay. Plant Cell 11:1267–1276
Zhang SC, Ghosh R, Jeske H (2002) Subcellular targeting domains of Abutilon mosaic geminivirus movement protein BC1. Arch Virol 147:2349–2363
Zhang SC, Wege C, Jeske H (2001) Movement proteins (BC1 and BV1) of Abutilon mosaic geminivirus are cotransported in and between cells of sink but not of source leaves as detected by green fluorescent protein tagging. Virology 290:249–260
Kalderon D, Roberts BL, Richardson WD, Smith AE (1984) A short amino acid sequence able to specify nuclear localization. Cell 39:499–509
Robbins J, Dilworth SM, Laskey RA, Digwall C (1991) Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64:615–623
Lazarowitz SG, Beachy RN (1999) Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell 11:535–548
Lazarowitz SG (1999) Probing plant cell structure and function with viral movement proteins. Curr Opin Plant Biol 2:332–338
Unseld S, Höhnle M, Ringel M, Frischmuth T (2001) Subcellular targeting of the coat protein of African cassava mosaic geminivirus. Virology 286:373–383
Kunik T, Palanichelvam K, Czosnek H, Citovsky V, Gafni Y (1998) Nuclear import of the capsid protein of tomato yellow leaf curl virus (TYLCV) in plant and insect cells. Plant J 13(3):393–399
Unseld S, Frischmuth T, Jeske H (2004) Short deletions in nuclear targeting sequences of African cassava mosaic virus coat protein prevent geminivirus twinned particle formation. Virology 318:89–100
Kikuno R, Toh H, Hayashida H, Miyata T (1984) Sequence similaritiy between putative gene products of geminiviral DNAs. Nature 308:562
Qin SW, Ward BM, Lazarowitz SG (1998) The bipartite geminivirus coat protein aids BR1 function in viral movement by affecting the accumulation of viral single-stranded DNA. J Virol 72:9247–9256
Saunders K, Salim N, Mali VR, Malathi VG, Briddon R, Markham PG, Stanley J (2002) Characterisation of Sri Lankan cassava mosaic virus and Indian cassava mosaic virus: evidence for acquisition of a DNA B component by a monopartite begomovirus. Virology 293:63–74
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Press, Cold Spring Harbor
Martins LGC, Raimundo GAS, Ribeiro NGA, Silva JCF, Euclydes NC, Loriato VAP, Duarte CEM, Fontes EPB (2020) A begomovirus nuclear shuttle protein-interacting immune hub: hijacking host transport activities and suppressing incompatible functions. Front Plant Sci 11:398. https://doi.org/10.3389/fpls.2020.00398
Mariano AC, Andrade MO, Santos AA, Carolino SM, Oliveira ML, Baracat-Pereira MC, Brommonshenkel SH, Fontes EP (2004) Identification of a novel receptor-like protein kinase that interacts with a geminivirus nuclear shuttle protein. Virology 318:24–31
Rojas MR, Noueiry AO, Lucas WJ, Gilbertson RL (1998) Bean dwarf mosaic geminivirus movement proteins recognize DNA in a form-and size- specific manner. Cell 95:105–113
Whittaker GR, Helenius A (1998) Nuclear import and export of viruses and virus genomes. Virology 246:1–23
Fontes EP, Santos AA, Luz DF, Waclawovsky AJ, Chory J (2004) The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev 18:2545–2556
Krapp S, Greiner E, Amin B, Sonnewald U, Krenz B (2017) The stress granule component G3BP is a novel interaction partner for the nuclear shuttle proteins of the nanovirus pea necrotic yellow dwarf virus and geminivirus abutilon mosaic virus. Virus Res 227:6–14. https://doi.org/10.1016/j.virusres.2016.09.021
Ward BM, Medville R, Lazarowitz SG, Turgeon R (1997) The geminivirus BL1 movement protein is associated with endoplasmic reticulum–derived tubules in developing phloem cells. J Virol 71:3726–3733
Maggioni C, Braakman I (2005) Synthesis and quality control of viral membrane proteins. Curr Top Microbiol Immunol 285:175–198
Boutin JA (1997) Myristoylation. Cell Signal 9:15–35
Mei Y, Wang Y, Hu T, Yang X, Lozano-Duran R, Sunter G, Zhou X (2018) Nucleocytoplasmic shuttling of geminivirus C4 protein mediated by phosphorylation and myristoylation is critical for viral pathogenicity. Mol Plant 11:1466–1481
Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10:524–530
Acknowledgements
The facilities provided at University of Stuttgart by Prof. Holger Jeske for part of this work are gratefully acknowledged. The study was funded by EU INCO-DEV Grant ICA-CT-2000-30001. BLP acknowledges Research Fellowship from Council for Scientific and Industrial Research, New Delhi, the DAAD Scholarship and the financial support of Vater-ünd-Sohn-Eiselen-Stiftung. Help in photography from Frau Sigrid Kober is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
ID and BLP conceived and designed the study, BLP performed the experiments and drafted the manuscript, ID supervised the study and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research did not involve any animal and/or human participants.
Additional information
Edited by Seung-Kook Choi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, B.L., Dasgupta, I. Characterization of the functional domains of nuclear shuttle protein (NSP) of Indian cassava mosaic virus using green fluorescent protein as reporter. Virus Genes 58, 308–318 (2022). https://doi.org/10.1007/s11262-022-01909-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-022-01909-5