Abstract
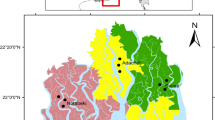
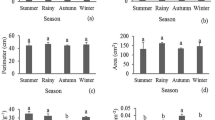
Mangrove tree species show plasticity in their leaf morphological traits in different salinity zones. However, leaf morphological plasticity and its causes in different salinity zones are incompletely understood. To understand the mechanism of plasticity, this study investigated the responses of three dominant tree species Sundri (Heritiera fomes), Gewa (Excoecaria agallocha) and Goran (Ceriops decandra) of the Sundarbans to the salinity gradients. A total of 17 leaf parameters were measured and quantified. All collected data were analyzed using univariate and multivariate statistical tools to investigate leaf morphological plasticity. A wide range of phenotypic plasticity was observed in all leaf parameters studied among the salinity zones of the Sundarbans. One-way ANOVA and Tukey’s posthoc test revealed significant differences (P < 0.05) in all leaf parameters among the salinity zones and confirming that there was a high degree of phenotypic plasticity among the salinity zones of the Sundarbans. Petiole length (PL), leaf area (LA) and leaf length/petiole length (LL/PL) showed high level of plasticity among the salinity zones of the Sundarbans for each species of Sundri, Gewa and Goran. Plasticity index (PI) was developed in this study for each species studied. High level of phenotypic plasticity in these leaf traits reflects fitness of these species to different saline environments. Our results provide clear evidence that all the leaf parameters measured for three tree species viz., Sundri, Gewa and Goran effectively utilizes a plastic strategy in different salinity zones in the Sundarbans. Morphological trait plasticity could serve as powerful biological indicators to predict the shift of leaf morphology in upcoming environmental change events like sea level rise and reduction of fresh water flow from upstream.





Similar content being viewed by others
References
Abbruzzese G, Beritognolo I, Muleo R, Piazzai M, Sabatti M, Mugnozza GS, Kuzminsky E (2009) Leaf morphological plasticity and stomatal conductance in three Populus alba L. genotypes subjected to salt stress. Environ Exp Bot 66:381–388
Alam MR, Mahmood H, Khushi MLR, Rahman MM (2018) Adaptive phenotypic plasticity of Avicennia officinalis L. across the salinity gradient in the Sundarbans of Bangladesh. Hydrobiologia 808:163–174. https://doi.org/10.1007/s10750-017-3420-z
Arrivabene HP, Souza I, Có WLO, Rodella RA, Wunderlin DA, Milanez CR (2014) Functional traits of selected mangrove species in Brazil as biological indicators of different environmental conditions. Sci Total Environ 476:496–504
Asaeda T, Barnuevo A (2019) Oxidative stress as an indicator of niche-width preference of mangrove Rhizophora stylosa. For Ecol Manag 432:73–82
Ball MC (2002) Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees 16:126–139
Banerjee K, Gatti RC, Mitra A (2017) Climate change-induced salinity variation impacts on a stenoecious mangrove species in the Indian Sundarbans. Ambio 46:492–499
Bruschi P, Grossoni P, Bussotti F (2003) Within-and among-tree variation in leaf morphology of Quercus petraea (Matt.) Liebl. Natural populations. Trees 17:164–172
Camilleri JC, Ribi G (1983) Leaf thickness of mangroves (Rhizophora mangle) growing in different salinities. Biotropica:139–141
Carins Murphy MR, Jordan GJ, Brodribb TJ (2012) Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ 35:1407–1418
Chaffey DR, Miller F, Sandom J (1985) A forest inventory of the Sundarbans, Bangladesh
Chevin LM, Collins S, Lefèvre F (2013) Phenotypic plasticity and evolutionary demographic responses to climate change: taking theory out to the field. Funct Ecol 27:967–979
Chitwood DH, Sinha NR (2016) Evolutionary and environmental forces sculpting leaf development. Curr Biol 26:R297–R306
Dangremond EM, Feller IC (2016) Precocious reproduction increases at the leading edge of a mangrove range expansion. Ecol Evol 6:5087–5092
Dasgupta S, Sobhan I, Wheeler D (2017) The impact of climate change and aquatic salinization on mangrove species in the Bangladesh Sundarbans. Ambio 46:680–694
Dickinson T, Parker W, Strauss R (1987) Another approach to leaf shape comparisons. Taxon 36:1–20
Duke N, Ball M, Ellison J (1998) Factors influencing biodiversity and distributional gradients in mangroves. Global Ecology & Biogeography Letters 7:27–47
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball M (2010) Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2:395–417
Fritz MA, Rosa S, Sicard A (2018) Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front Genet 9:478
Geekiyanage N, Goodale UM, Cao K, Kitajima K (2018) Leaf trait variations associated with habitat affinity of tropical karst tree species. Ecol Evol 8:286–295
Givnish TJ (1987) Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol 106:131–160
Gomes PIA, Asaeda T (2009) Spatial and temporal heterogeneity of Eragrostis curvula in the downstream flood medow of a regulated river. Ann Limnol-Int J Lim 45:181–193
Granata MU, Bracco F, Catoni R (2020) Phenotypic plasticity of two invasive alien plant species inside a deciduous forest in a strict nature reserve in Italy. J Sustain For 39:346–364
Guimarães ZTM, dos Santos VAHF, Nogueira WLP, de Almeida Martins NO, Ferreira MJ (2018) Leaf traits explaining the growth of tree species planted in a central Amazonian disturbed area. For Ecol Manag 430:618–628
Hay A, Tsiantis M (2006) The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet 38:942
Hoppe-Speer SC, Adams JB, Rajkaran A, Bailey D (2011) The response of the red mangrove Rhizophora mucronata lam. To salinity and inundation in South Africa. Aquat Bot 95:71–76
Hoque AF, Datta DK (2005) The mangroves of Bangladesh. Int J Ecol Environ Sci 31:245–253
Hossain M, Siddique MRH, Bose A, Limon SH, Chowdhury MRK, Saha S (2012) Allometry, above-ground biomass and nutrient distribution in Ceriopsdecandra (Griffith) ding Hou dominated forest types of the Sundarbans mangrove forest, Bangladesh. Wetl Ecol Manag 20:539–548
Hossain M, Siddique MRH, Abdullah SR, Saha S, Ghosh DC, Rahman MS, Limon SH (2014) Nutrient dynamics associated with leaching and microbial decomposition of four abundant mangrove species leaf litter of the Sundarbans, Bangladesh. Wetlands 34:439–448
Hossain M, Siddique MRH, Saha S, Abdullah SR (2015) Allometric models for biomass, nutrients and carbon stock in Excoecaria agallocha of the Sundarbans, Bangladesh. Wetl Ecol Manag 23:765–774
Iftekhar M, Saenger P (2008) Vegetation dynamics in the Bangladesh Sundarbans mangroves: a review of forest inventories. Wetl Ecol Manag 16:291–312
Ishii HR, S-i H, Noguchi Y, Azuma W (2018) Variation of intra-crown leaf plasticity of Fagus crenata across its geographical range in Japan. For Ecol Manag 429:437–448
Khan MNI, Suwa R, Hagihara A (2005) Allometric relationships for estimating the aboveground phytomass and leaf area of mangrove Kandelia candel (L.) Druce trees in the Manko wetland, Okinawa Island, Japan. Trees 19:266–272
Klančnik K, Gaberščik A (2015) Leaf spectral signatures differ in plant species colonizing habitats along a hydrological gradient. J Plant Ecol 9:442–450
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass, and productivity of mangrove forests: a review. Aquat Bot 89:128–137. https://doi.org/10.1016/j.aquabot.2007.12.006
Krauss KW, Allen JA (2003) Factors influencing the regeneration of the mangrove Bruguiera gymnorrhiza (L.) Lamk. On a tropical Pacific island. For Ecol Manag 176:49–60
Krauss KW, Lovelock CE, McKee KL, López-Hoffman L, Ewe SM, Sousa WP (2008) Environmental drivers in mangrove establishment and early development: a review. Aquat Bot 89:105–127
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer Science & Business Media,
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Lira-Medeiros CF, Parisod C, Fernandes RA, Mata CS, Cardoso MA, Ferreira PCG (2010) Epigenetic variation in mangrove plants occurring in contrasting natural environment. PLoS One 5
Lopez-Hoffman L, Anten NP, Martinez-Ramos M, Ackerly DD (2007) Salinity and light interactively affect neotropical mangrove seedlings at the leaf and whole plant levels. Oecologia 150:545
Lovelock CE, Feller IC, Ellis J, Schwarz AM, Hancock N, Nichols P, Sorrell B (2007) Mangrove growth in New Zealand estuaries: the role of nutrient enrichment at sites with contrasting rates of sedimentation. Oecologia 153:633–641
McKee KL (1995) Interspecific variation in growth, biomass partitioning, and defensive characteristics of neotropical mangrove seedlings: response to light and nutrient availability. Am J Bot 82:299–307
Mollick AS, Shimoji H, Denda T, Yokota M, Yamasaki H (2011) Croton Codiaeum variegatum (L.) Blume cultivars characterized by leaf phenotypic parameters. Sci Hortic 132:71–79
Nackley LL, Kim SH (2015) A salt on the bioenergy and biological invasions debate: salinity tolerance of the invasive biomass feedstock a rundo donax. GCB Bioenergy 7:752–762
Naidoo G (2010) Ecophysiological differences between fringe and dwarf Avicennia marina mangroves. Trees 24:667–673
Naidoo G (2016) The mangroves of South Africa: an ecophysiological review. S Afr J Bot 107:101–113
Nakayama H, Sinha NR, Kimura S (2017) How do plants and phytohormones accomplish heterophylly, leaf phenotypic plasticity, in response to environmental cues. Front Plant Sci 8:1717
Nandy P, Das S, Ghose M, Spooner-Hart R (2007) Effects of salinity on photosynthesis, leaf anatomy, ion accumulation and photosynthetic nitrogen use efficiency in five Indian mangroves. Wetl Ecol Manag 15:347–357
Nasrin S, Hossain M, Rahman MM (2019) Adaptive responses to salinity: nutrient resorption efficiency of Sonneratia apetala (Buch.-ham.) along the salinity gradient in the Sundarbans of Bangladesh. Wetl Ecol Manag 27:343–351
Navas M-L, Garnier E (2002) Plasticity of whole plant and leaf traits in Rubia peregrina in response to light, nutrient and water availability. Acta Oecol 23:375–383. https://doi.org/10.1016/S1146-609X(02)01168-2
Neale DB, Kremer A (2011) Forest tree genomics: growing resources and applications. Nat Rev Genet 12:111
Nicotra AB, Atkin OK, Bonser SP, Davidson AM (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692
Peppe DJ, Royer DL, Cariglino B, Oliver SY (2011) Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol 190:724–739
Pérez-Harguindeguy N, Diaz S, Gamier E, Lavorel S (2013) New handbook for stand-ardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234
Petruzzellis F, Peng G, Tyree MT, Tonet V, Savi T (2019) Plasticity of functional traits of tree of heaven is higher in exotic than in native habitats. Trees 33:411–420
Pigliucci M, Kolodynska A (2002) Phenotypic plasticity and integration in response to flooded conditions in natural accessions of Arabidopsis thaliana (L.) Heynh (Brassicaceae). Ann Bot 90:199–207
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/
Rahman MM, Khan NIM, Hoque FAK, Ahmed I (2015) Carbon stock in the Sundarbans mangrove forest: spatial variations in vegetation types and salinity zones. Wetl Ecol Manag 23:269–283. https://doi.org/10.1007/s11273-014-9379-x
Ramírez-Valiente JA, Sánchez-Gómez D, Aranda I, Valladares F (2010) Phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiol 30:618–627
Reef R, Feller IC, Lovelock CE (2010) Nutrition of mangroves. Tree Physiol 30:1148–1160
Rozendaal D, Hurtado V, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct Ecol 20:207–216
Sardans J, Peñuelas J, Rodà F (2006) Plasticity of leaf morphological traits, leaf nutrient content, and water capture in the Mediterranean evergreen oak Quercus ilex subsp. ballota in response to fertilization and changes in competitive conditions. Ecoscience 13:258–270
Sarker SK, Reeve R, Thompson J, Paul NK, Matthiopoulos J (2016) Are we failing to protect threatened mangroves in the Sundarbans world heritage ecosystem? Sci Rep 6:1–12
Sarker SK, Matthiopoulos J, Mitchell SN, Ahmed ZU, Al Mamun MB, Reeve R (2019) 1980s–2010s: the world’s largest mangrove ecosystem is becoming homogenous. Biol Conserv 236:79–91
Schmitz N, Jansen S, Verheyden A, Kairo JG, Beeckman H, Koedam N (2007) Comparative anatomy of intervessel pits in two mangrove species growing along a natural salinity gradient in Gazi Bay, Kenya. Ann Bot 100:271–281
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Shi G, Cai Q (2009) Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environ Exp Bot 67:112–117
Siddiqi N (2001) Mangrove forestry in Bangladesh. Institute of Forestry & Environmental Sciences, University of Chittagong,
Siddique MRH, Saha S, Salekin S, Mahmood H (2017) Salinity strongly drives the survival, growth, leaf demography, and nutrient partitioning in seedlings of Xylocarpus granatum J. König. iForest-biogeosciences and. Forestry 10:851
Simpson LT, Feller IC, Chapman SK (2013) Effects of competition and nutrient enrichemnt on Avicennia germinans in the salt marsh-mangrove ecotone. Aquat Bot 104:55–59
Sultan SE (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5:537–542
Sun P, Jia H, Cheng X, Zhang Y, Li J, Zhang L, Lu M, Zhang J, Hu J (2020) Genetic architecture of leaf morphological and physiological traits in a Populus deltoides ‘Danhong’× P. simonii ‘Tongliao1’pedigree revealed by quantitative trait locus analysis. Tree Genet Genomes 16:1–14
Tomlinson PB (2016) The botany of mangroves. Cambridge University Press, Cambridge
Traiser C, Klotz S, Uhl D, Mosbrugger V (2005) Environmental signals from leaves–a physiognomic analysis of European vegetation. New Phytol 166:465–484
Tsukaya H (2002a) Leaf development. The Arabidopsis Book/American Society of Plant Biologists 1
Tsukaya H (2002b) The leaf index: heteroblasty, natural variation, and the genetic control of polar processes of leaf expansion. Plant Cell Physiol 43:372–378
Tsukaya H (2013) Leaf development. The Arabidopsis Book/American Society of Plant Biologists 11
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116
Viscosi V (2015) Geometric morphometrics and leaf phenotypic plasticity: assessing fluctuating asymmetry and allometry in European white oaks (Quercus). Bot J Linn Soc 179:335–348
Vovides AG, Vogt J, Kollert R, Berger U, Grueters U, Peters R, Lara-Dominguez AL, Lopez-Portillo J (2014) Morphological plasticity in mangrove trees: salinity-related changes in the allometry of Avicennia germinans. Trees 28:1413–1425
Wahid SM, Babel MS, Bhuiyan AR (2007) Hydrologic monitoring and analysis in the Sundarbans mangrove ecosystem, Bangladesh. J Hydrol 332:381–395
Wang W, Yan Z, You S, Zhang Y, Chen L, Lin G (2011) Mangroves: obligate or facultative halophytes? A review. Trees 25:953–963
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wright I, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Funct Ecol 15:351–359
Xu F, Guo W, Xu W, Wang R (2008) Habitat effects on leaf morphological plasticity. Acta Biol Cracoviensia Ser Bot 50:19–26
Xu F, Guo W, Xu W, Wei Y, Wang R (2009) Leaf morphology correlates with water and light availability: what consequences for simple and compound leaves? Prog Nat Sci 19:1789–1798
Yuan T, Masaaki H, Wenxuan L (2016) Ggfortify: unified Interface to visualize statistical result of popular R packages. The R Journal 8(2):478–489
Acknowledgements
We thank to Forest Department officials of the Sundarbans East and West Forest Division for their help during leaf sample collection. We are grateful to Professor Dr. Md. Iftekhar Shams for providing facility to carry out lab experiments in Nano Biomaterial Lab of Forestry and Wood Technology Discipline, Khulna University. This work was supported by University Grants Commission of Bangladesh (Project ID: UGC/SciTech/Agri (Crop-47)/2017/4915). Thanks to the excellent Editor and anonymous Reviewers for their valuable comments to improve the quality of this manuscript.
Author information
Authors and Affiliations
Contributions
ASM conceived the idea and designed the experiment. RS performed the field data collection and laboratory experiment. ASM, MNIK and MSA analyzed the data. ASM drafted the first version of the manuscript. MNIK helped with manuscript revisions. All authors read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mollick, A.S., Sultana, R., Azad, M.S. et al. Leaf morphological plasticity in three dominant tree species in the Sundarbans mangrove forest of Bangladesh in different salinity zones. Wetlands Ecol Manage 29, 265–279 (2021). https://doi.org/10.1007/s11273-020-09782-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-020-09782-5