Abstract
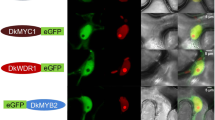
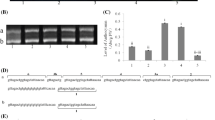
MYB-bHLH-WD40 (MBW) ternary complexes composed of MYB domain proteins, basic helix-loop-helix (bHLH) transcription factors and WD40-repeat proteins are involved in the transcriptional regulation of anthocyanin and proanthocyanidin (PA) biosynthesis pathways in distinct plant species. PAs are the compounds responsible for the strong astringent taste of persimmon fruit. DkMYB2 and DkMYB4 (MYB type) and DkMYC1 (bHLH type) genes from persimmon have been postulated to regulate the biosynthesis of PAs in fruit. We have identified a WD40-repeat gene from persimmon (DkWDR1) coding for a protein highly similar to TTG1 from Arabidopsis thaliana and other WD40-repeat components of distinct MBW complexes. DkWDR1 expression was detected in different tissues and was essentially constant during fruit development. MYB-like proteins DkMYB2 and DkMYB4 interacted with both DkWDR1 and DkMYC1 in the yeast two-hybrid system. The sequence of DkWDR1 and the analysis of yeast two-hybrid interactions with known regulators of PA biosynthesis support the occurrence of MYB-bHLH-WD40 (MBW) complexes in persimmon, to regulate PA accumulation in fruit.








Similar content being viewed by others
References
Abrahams S, Tanner GJ, Larkin PJ, Ashton AR (2002) Identification and biochemical characterization of mutants in the proanthocyanidin pathway in Arabidopsis. Plant Physiol 130:561–576
Akagi T, Ikegami A, Suzuki Y, Yoshida J, Yamada M, Sato A, Yonemori K (2009a) Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit. Planta 230:899–915
Akagi T, Ikegami A, Tsujimoto T, Kobayashi S, Sato A, Kono A, Yonemori K (2009b) DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol 151:2028–2045
Akagi T, Ikegami A, Yonemori K (2010) DkMyb2 wound-induced transcription factor of persimmon (Diospyros kaki Thunb.), contributes to proanthocyanidin regulation. Planta 232:1045–1059
Akagi T, Katayama-Ikegami A, Yonemori K (2011a) Proanthocyanidin biosynthesis of persimmon (Diospyros kaki Thunb.) fruit. Sci Hortic 130:373–380
Akagi T, Tsujimoto T, Ikegami A, Yonemori K (2011b) Effects of seasonal temperature changes on DkMyb4 expression involved in proanthocyanidin regulation in two genotypes of persimmon (Diospyros kaki Thunb.) fruit. Planta 233:883–894
Akagi T, Katayama-Ikegami A, Kobayashi S, Sato A, Kono A, Yonemori K (2012) Seasonal abscisic acid signal and a basic leucine zipper transcription factor, DkbZIP5, regulate proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol 158:1089–1102
An XH, Tian Y, Chen KQ, Wang XF, Hao YJ (2012) The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation. J Plant Physiol 169:710–717
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Arnal L, del Río MA (2004) Quality of persimmon fruit cv. ‘Rojo Brillante’ during storage at different temperatures. Span J Agric Res 2:243–247
Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, Joshi SS, Pruess HG (2000) Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology 148:187–197
Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39:366–380
Bellini E (2002) Cultural practices for persimmon production. In: Bellini E, Giordani E (eds) First mediterranean symposium on persimmon. CIHEAM, Zaragoza, pp 39–52
Brueggemann J, Weisshaar B, Sagasser M (2010) A WD40-repeat gene from Malus x domestica is a functional homologue of Arabidopsis thaliana TRANSPARENT TESTA GLABRA1. Plant Cell Rep 29:285–294
Brunner AM, Yakovlev IA, Strauss SH (2004) Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol 4:14
Cone KC (2007) Anthocyanin synthesis in maize aleurone tissue. Plant Cell Monogr 8:121–139
de Vetten N, Quattrocchio F, Mol J, Koes R (1997) The an11 locus controlling flower pigmentation in petunia encodes a novel WD-repeat protein conserved in yeast, plants, and animals. Genes Dev 11:1422–1434
Dixon RA, Xie D-Y, Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytol 165:9–28
Gambino G, Perrone I, Gribaudo I (2008) A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19:520–525
Georgiev V, Ananga A, Tsolova V (2014) Recent advances and uses of grapes flavonoids and nutraceuticals. Nutrients 6:391–415
Goff SA, Cone KC, Chandler VL (1992) Functional analysis of the transcriptional activator encoded by the maize B gene: evidence for a direct functional interaction between two classes of regulatory proteins. Genes Dev 6:864–875
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827
Harborne JB, Grayer RJ (1993) Flavonoids and insects. In: Harborne JB (ed) The flavonoids: advances in research since 1986. Chapman and Hall, London, pp 589–618
Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V (2011) Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot 62:2465–2483
Humphries JA, Walker AR, Timmis JN, Orford SJ (2005) Two WD-repeat genes from cotton are functional homologues of the Arabidopsis thaliana TRANSPARENT TESTA GLABRA1 (TTG1) gene. Plant Mol Biol 57:67–81
Ikegami A, Kitajima A, Yonemori K (2005) Inhibition of flavonoid biosynthetic gene expression coincides with loss of astringency in pollination constant, non-astringent (PCNA)-type persimmon fruit. J Hortic Sci Biotechnol 80:225–228
Jende-Strid B (1993) Genetic control of flavonoid biosynthesis in barley. Hereditas 119:187–204
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57:405–430
Liu X, Feng C, Zhang M, Yin X, Xu C, Chen K (2013) The MrWD40-1 gene of Chinese bayberry (Myrica rubra) interacts with MYB and bHLH to enhance anthocyanin accumulation. Plant Mol Biol Report 31:1474–1484
Manach C, Williamson G, Morand C, Scaibert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81(1 Suppl):230S–242S
Marles MAS, Ray H, Gruber MY (2003) New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 64:367–383
Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L (2000) The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12:1863–1878
Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L (2001) The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13:2099–2114
Payne CT, Zhang F, Lloyd AM (2000) GL3 encodes a bHLH protein that regulates trichome development in arabidopsis through interaction with GL1 and TTG1. Genetics 156:1349–1362
Salvador A, Arnal L, Besada C, Larrea V, Quiles A, Pérez-Munuera I (2007) Physiological and structural changes during ripening and deastringency treatment of persimmon cv. ‘Rojo Brillante’. Postharvest Biol Technol 46:181–188
Scalbert A (1991) Antimicrobial properties of tannins. Phytochemistry 30:3875–3883
Schaart JG, Dubos C, Romero De La Fuente I, van Houwelingen AM, de Vos RC, Jonker HH, Xu W, Routaboul JM, Lepiniec L, Bovy AG (2013) Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol 197:454–467
Sompornpailin K, Makita Y, Yamazaki M, Saito K (2002) A WD-repeat-containing putative regulatory protein in anthocyanin biosynthesis in Perilla frutescens. Plant Mol Biol 50:485–495
Spelt C, Quattrocchio F, Mol J, Koes R (2002) ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell 14:2121–2135
Su F, Hu J, Zhang Q, Luo Z (2012) Isolation and characterization of a basic Helix–Loop–Helix transcription factor gene potentially involved in proanthocyanidin biosynthesis regulation in persimmon (Diospyros kaki Thunb.). Sci Hortic 136:115–121
Taheri A, Jayasankar S, Cline JA, Raizada MN, Pauls PK (2012) A WD-repeat gene from peach (Prunus persica L.) is a functional ortholog of Arabidopsis thaliana TRANSPARENT TESTA GLABRA1. In Vitro Cell Dev Biol Plant 48:23–29
Taira S (1995) Astringency in persimmon. In: Linskens HF, Jackson JF (eds) Fruit analysis. Springer, Berlin, pp 97–110
Tamura M, Tao R, Yonemori K, Untunomia N, Sugiura A (1998) Ploidy level and genome size of several Diospyros species. J Jpn Soc Hortic Sci 67:306–312
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR (2003) Proanthocyanidin biosynthesis in plants: purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J Biol Chem 278:31647–31656
Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11:1337–1350
Wang Y, Zhang QL, Luo ZR (2010) Isolation and expression of gene encoding leucoanthocyanidin reductase from Diospyros kaki during fruit development. Biol Plant 54:707–710
Wang Y, Jiang F, Zhuo Z, Wu X-H, Wu Y-D (2013) A method for WD40 repeat detection and secondary structure prediction. PLoS ONE 8:e65705
Winkel-Shirley B (2001) Flavonoid biosynthesis. a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Xie DY, Sharma SB, Paiva NL, Ferreira D, Dixon RA (2003) Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299:396–399
Xu W, Grain D, Bobet S, Le Gourrierec J, Thévenin J, Kelemen Z, Lepiniec L, Dubos C (2014) Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol 202:132–144
Xu W, Dubos C, Lepiniec L (2015) Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 20:176–185
Yonemori K, Matsushima J (1985) Property of development of the tannin cells from nonastringent and astringent type fruits of Japanese persimmon (Diospyros kaki) and its relationship to natural astringency. J Jpn Soc Hortic Sci 54:201–208
Yonemori K, Matsushima J (1987) Changes in tannin cell morphology with growth and development of Japanese persimmon fruit. J Am Soc Hortic Sci 112:818–821
Yonemori K, Sugiura A, Yamada M (2000) Persimmon genetics and breeding. Plant Breed Rev 19:191–225
Zhuang D, Kitajima A, Ishida M, Sobajima Y (1990) Chromosome number of Diospyros kaki cultivars. J Jpn Soc Hortic Sci 59:289–297
Acknowledgments
This work was funded by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA)-FEDER (grant number RF2013-00043-C02-02) and the Ministry of Science and Innovation of Spain (grant number AGL2010-20595). FG-M and ALL were funded by a fellowship co-financed by the European Social Fund and the Instituto Valenciano de Investigaciones Agrarias (IVIA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. In addition the research is not involving human participants or animals.
Data archiving statement
NCBI GenBank accession numbers are provided in Online Resource Table S3.
Additional information
Communicated by A.M. Dandekar
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource Table S1
Primers used in this study. Primers obtained or modified from Akagi et al. (2009b) are labelled with (*). (PDF 46 kb)
Online Resource Table S2
Stability index of gene expression. (PDF 27 kb)
Online Resource Table S3
Allele composition of DkWDR1 in ‘Hachiya’ cultivar. (PDF 32 kb)
Online Resource Fig. S1
Alignment of DkWDR1 alleles and deduced proteins. (PDF 56 kb)
Online Resource Fig. S2
Polymorphisms of DkWDR1 in the non-astringent variety ‘Hana Fuyu’ by sequencing of a pooled PCR product. (PDF 46 kb)
Rights and permissions
About this article
Cite this article
Naval, M.d.M., Gil-Muñoz, F., Lloret, A. et al. A WD40-repeat protein from persimmon interacts with the regulators of proanthocyanidin biosynthesis DkMYB2 and DkMYB4. Tree Genetics & Genomes 12, 13 (2016). https://doi.org/10.1007/s11295-016-0969-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-016-0969-z