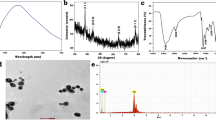
Abstract
The proliferation of silver nanoparticle (AgNP) production and use owing to their antimicrobial properties justifies the need to examine the resulting environmental impacts. The discharge of biocidal nanoparticles to water bodies may pose a threat to aquatic species. This study evaluated the effects of citrate-coated AgNPs on the standardized test organism Daphnia magna Straus clone MBP996 by means of biochemical biomarker response. AgNP toxicity was compared against the toxic effect of Ag+. The toxicity endpoints were calculated based upon measured Ag concentrations in exposure media. For AgNPs, the NOAEC and LOAEC values at 48 h were 5 and 7 μg Ag/L, respectively, while these values were 0.5 and 1 μg Ag/L, respectively, for Ag+. The EC50 at 48 h was computed to be 12.4 ± 0.6 and 2.6 ± 0.1 μg Ag/L for AgNPs and Ag+, respectively, with 95 % confidence intervals of 12.1–12.8 and 2.3–2.8 μg Ag/L, respectively. These results indicate significant less toxicity of AgNP compared to free Ag+ ions. Five biomarkers were evaluated in Daphnia magna neonates after acute exposure to Ag+ or AgNPs, including glutathione (GSH) level, reactive oxygen species (ROS) content, and catalase (CAT), acetylcholinesterase (AChE), and superoxide dismutase (SOD) activity. AgNPs induced toxicity and oxidative stress responses in D. magna neonates at tenfold higher concentrations than Ag. Biochemical methods revealed a clear increase in AChE activity, decreased ROS level, increased GSH level and CAT activity, but no significant changes in SOD activity. As Ag+ may dissolve from AgNPs, these two types of Ag could act synergistically and produce a greater toxic response. The observed remarkably high toxicity of AgNPs (in the parts-per-billion range) to crustaceans indicates that these organisms are a vulnerable link in the aquatic food chain with regard to contamination by nanosilver.

ᅟ




Similar content being viewed by others
References
Allen HJ, Impellitteri CA, Macke DA, Heckman JL, Poynton HC, Lazorchak JM, Govindaswamy S, Roose DL, Nadagouda MN (2010) Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna. Environ Toxicol Chem 29:2742–2750
Asghari S, Johari SA, Lee JH, Kim YS, Jeon YB, Choi HJ, Moon MC, Yu IJ (2012) Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J Nanobiotechnol 10:14
Barata C, Varo I, Navarro JC, Arun S, Porte C (2005) Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comp Biochem Physiol C Toxicol Pharmacol 140:175–186
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17:387–395
Beauvais SL, Jones SB, Parris JT, Brewer SK, Little EE (2001) Cholinergic and behavioural neurotoxicity of carbaryl and cadmium to larval rainbow trout (Oncorhynchus mykiss). Ecotoxicol Environ Saf 49:84–90
Blinova I, Niskanen J, Kajankari P, Kanarbik L, Käkinen A, Tenhu H, Penttinen O-P, Kahru A (2013) Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus. Environ Sci Pollut Res 20:3456–3463
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200
Chen J, Rogers SC, Kavdia M (2013) Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann Biomed Eng 41:327–337
Choi O, Clevenger TE, Deng B, Surampalli RYL, Ross J, Hu Z (2009) Role of sulfide and ligand strength in controlling nanosilver toxicity. Water Res 43:1879–1886
Dalai S, Pakrashi S, Chandrasekaran N, Mukherjee A (2013) Acute toxicity of TiO2 nanoparticles to Ceriodaphnia dubia under visible light and dark conditions in a freshwater system. PLoS ONE 8:e62970
Devi M, Fingerman M (1995) Inhibition of acetylcholinesterase activity in the central nervous system of red swamp cray fish, Procambarus clarkia by mercury, cadmium and lead. Bull Environ Contam Toxicol 55:746–750
Diamantino TC, Guilhermino L, Almeida E, Soares AMVM (2000) Toxicity of sodium molybdate and sodium dichromate to Daphnia magna Straus evaluated in acute, chronic, and acetylcholinesterase inhibition tests. Ecotoxicol Environ Saf 45:253–259
Dominguez GA, Lohse SE, Torelli MD, Murphy CJ, Hamers RJ, Orr G, Klaper RD (2015) Effects of charge and surface ligand properties of nanoparticles on oxidative stress and gene expression within the gut of Daphnia magna. Aquat Toxicol 162:1–9
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
European Parliament and European Council, Directive 2006/121/EC. 2006. Off J Eur Union 561(L396):850.
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behavior and effects in the aquatic environment. Environ Int 37:517–531
Fan WH, Wang XL, Cui M, Zhang D, Zhang Y, Yu T, Guo L (2012) Differential oxidative stress of octahedral and cubic Cu2O micro/nanocrystals to Daphnia magna. Environ Sci Technol 46:10255–10262
Gaiser BK, Biswas A, Rosenkranz P, Jepson MA, Lead JR, Stone V, Tyler CR, Fernandes TF (2011) Effects of silver and cerium dioxide micro- and nano-sized particles on Daphnia magna. J Environ Monit 13:1227–1235
Gallegos MEH, Zannatha MMI, Osornio EG, Sanches AS, Rio FAP (2001) Immediate and delayed effects of lead on AChE, GSH-T and thiols in the substantia nigra, neostriatum and cortex of the rat brain. J App Toxicol 21:397–401
Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27:1972–1978
Harris CA, Scott AP, Johnson AC, Panter GH, Sheahan D, Roberts M, Sumpter JP (2014) Principles of sound ecotoxicology. Environ Sci Technol 48:3100–3111
Hoheisel SM, Diamond S, Mount D (2012) Comparison of nanosilver and ionic silver toxicity in Daphnia magna and Pimephales promelas. Environ Toxicol Chem 31:2557–2563
Jemec A, Tišler T, Drobne D, Sepčić K, Jamnik P, Roš M (2008) Biochemical biomarkers in chronically metal-stressed daphnids. Comp Biochem Physiol Part C 147:61–68
Kahru A, Dubourguier HC (2010) From ecotoxicology to nanoecotoxicology. Toxicology 269:105–119
Kahru A, Dubourguier HC, Blinova I, Ivask I, Kasemets K (2008) Biotests and biosensors for ecotoxicology of metal oxide nanoparticles: a minireview. Sensors 8:5153–5170
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Klimisch H-J, Andreae M, Tillmann U (1997) A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol 25:1–5
Li H, Xia H, Wang D, Tao X (2013) Simple synthesis of monodisperse, quasi-spherical, citrate-stabilized silver nanocrystals in water. Langmuir 29:5074–5079
Marklund SL, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Matés JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Mehlen P, Kretz-Remy C, Préville X, Arrigo AP (1996) Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J 15:2695–2706
Mwaanga P, Carraway ER, van den Hurk P (2014) The induction of biochemical changes in Daphnia magna by CuO and ZnO nanoparticles. Aquat Toxicol 150:201–209
Najimi S, Bouhaimi A, Daubèze M, Zekhnini A, Pellerin J, Narbone JF, Moukrim A (1997) Use of acetylcholinesterase in Perna perna and Mytilus galloprovincialis as a biomarker of pollution in Agadir Marine Bay (South of Morocco). Bull Environ Contamin Toxicol 58:901–908
OECD Guidelines for the testing of chemicals (2004) Section 2: Effects on Biotic Systems. Test No. 202: Daphnia sp. Acute immobilisation test. Organisation for Economic Co-operation and Development, Paris, France
OECD Guidelines for the testing of chemicals (2011) Section 2: Effects on Biotic Systems. Test No. 201: Freshwater Alga and Cyanobacteria, growth inhibition test. Organisation for Economic Co-operation and Development, Paris, France
Pakrashi S, Dalai S, Humayun A, Chakravarty S, Chandrasekaran N, Mukherjee A (2013) Ceriodaphnia dubia as a potential bio-indicator for assessing acute aluminum oxide nanoparticle toxicity in fresh water environment. PLoS ONE 8:e74003
Romani R, Antognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E, Rosi G (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem-Bio Inter 145:321–329
Römer I, Gavin AJ, White TA, Merrifield RC, Chipman JK, Viant MR, Lead JR (2013) The critical importance of defined media conditions in Daphnia magna nanotoxicity studies. Toxicol Lett 223:103–108
Šinko G, Vinković Vrček I, Goessler W, Leitinger G, Dijanošić A, Miljanić S (2013) Alteration of cholinesterase activity as possible mechanism of silver nanoparticle toxicity. Environ Sci Pollut Res 21:1391–1400
Stensberg MC, Madangopal R, Yale G, Wei Q, Ochoa-Acuña H, Wei A, Mclamore ES, Rickus J, Porterfield DM, Sepúlveda MS (2014) Silver nanoparticle-specific mitotoxicity in Daphnia magna. Nanotoxicology 8:833–842
Strużyński W, Dąbrowska-Bouta B, Grygorowicz T, Ziemińska E, Strużyńska L (2014) Markers of oxidative stress in hepatopancreas of crayfish (Orconectes limosus, raf) experimentally exposed to nanosilver. Environ Toxicol 29:1283–1291
Suresh A, Sivaramakrishna B, Victoriamma PC, Radhakrishnaiah K (1992) Comparative study on the inhibition of acetylcholinesterase activity in the freshwater fish Cyprinus carpio by mercury and zinc. Biochem Int 26:367–375
Toumi H, Boumaiza M, Millet M, Radetski CM, Felten V, Ferard JF (2015) Is acetylcholinesterase a biomarker of susceptibility in Daphnia magna (Crustacea, Cladocera) after deltamethrin exposure? Chemosphere 120:351–356
Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T (2002) In vivo and in vitro effects of aluminium on the activity of mouse brain acetylcholinesterase. Brain Res Bull 59:41–45
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:885–892
Zou X, Shi J, Zhang H (2014) Coexistence of silver and titanium dioxide nanoparticles: enhancing or reducing environmental risks? Aquat Toxicol 154:168–175
Acknowledgments
This research was supported by the Institute of Public Health “Dr. Andrija Štampar” and the Institute for Medical Research and Occupational Health, Analytical Toxicology and Mineral Metabolism Unit. Special thanks to Doc. Dr. Marija Ćurlin, for providing access to the TEM Analysis Services Lab, for support with NP characterization.
Conflict of interest
The authors declare that there is no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Hutchinson
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Size distribution by volume of citrate-coated AgNPs in ultrapure water (Fig. S1) and standard culture medium used for D. magna (Fig. S2) (DOCX 108 kb)
Rights and permissions
About this article
Cite this article
Ulm, L., Krivohlavek, A., Jurašin, D. et al. Response of biochemical biomarkers in the aquatic crustacean Daphnia magna exposed to silver nanoparticles. Environ Sci Pollut Res 22, 19990–19999 (2015). https://doi.org/10.1007/s11356-015-5201-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5201-4