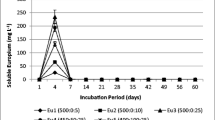
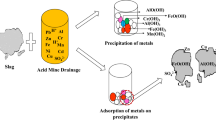
Abstract
Passive systems are often used for the treatment of acid mine drainage (AMD) on closed and abandoned mine sites. Metal-rich residues (solid precipitates) with variable chemical composition and physical properties can be generated. Their characterization is required to better anticipate the potential fate, including stability for disposal, potential recovery, or reuse. The present study evaluated the leaching potential of solids from a laboratory passive multi-step treatment for Fe-rich AMD (2350 ± 330 mg/L Fetot, 0.7 ± 0.4 mg/L Ni, 0.2 ± 3.0 mg/L Zn, and 5073 ± 407 mg/L SO42-, at pH 3.04 ± 0.45). To do so, post-treatment solids from three units (Fe-pretreatment reactor (50% wood chips and 50% wood ash, WA50), passive biochemical reactor, PBR for SO42− treatment (30% inorganic materials, 70% organic substrate), and polishing reactor (50% calcite and 50% wood chips, C50)) of a pilot laboratory treatment system were sampled. Physicochemical and mineralogical characterization, as well as static leaching tests were then performed. Results showed that all solids had high neutralizing potential, while high inorganic carbon was found in C50. Moreover, high metal concentrations were found in WA50. Metals and sulfates in all solids precipitated in the form of oxyhydroxides, oxy-hydroxy-sulfates, carbonates, sulfides, sulfate, and native sulfur. The Fe was not found as problematic contaminant in solids, but it was in AMD. However, a probable generation of contaminated neutral drainage by Ni and Zn could occur from WA50. The C50 had the highest acid neutralizing capacity and could better resist to acid aggression relative to solids from PBR and WA50. The PBR and C50 solids were considered as non-hazardous towards regulation’s limits and a potential co-disposal with municipal wastes could be a storage option. Further studies should be undertaken by testing other leaching and kinetic tests to assess long-term metal stability.








Similar content being viewed by others
Abbreviations
- AMD:
-
Acid mine drainage
- ANC :
-
Acid neutralizing capacity
- DAS:
-
Dispersed alkaline substrate
- PBR:
-
Passive biochemical reactor
References
Acero P, Ayora C, Torrento C, Nieto JM (2006) The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim Cosmochim Acta 70:4130–4139
APHA (American Public Health Association) (2012) In: Greenberg A (ed) Alkalinity titration, Standard methods for the examination of water and wastewater, 22nd edn, Washington DC, USA
Asta MP, Cama J, Martínez M, Giménez J (2009) Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. J Hazard Mater 171:965–972
ASTM (American Society for Testing and Materials) (1995) Standard test method for pH of soils. In: Annual Book of ASTM Standards D4972-95a, Washington, DC
Ayora C, Caraballo MA, Macías F, Rötting TS, Carrera J, Nieto JM (2013) Acid mine drainage in the Iberian Pyrite Belt: 2. Lessons learned from recent passive remediation experiences. Environ Sci Pollut Res 20:7837–7853
Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M (1996) Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim Cosmochim Acta 60(12):2111–2121
Brouwer H, Murphy TP (1994) Diffusion method for the determination of acid-volatile sulfides (AVS) in sediment. Environ Toxicol Chem 13:1273–1275
Calugaru IL, Neculita CM, Genty T, Bussière B, Potvin B (2016) Performance of thermally activated dolomite for the treatment of Ni and Zn in contaminated neutral drainage. J Hazard Mater 310:48–55
Cappuyns V, Swennen R (2008) The application of pHstat leaching tests to assess the pH-dependent release of trace metals from soils, sediments and waste materials. J Hazard Mater 158:185–195
Caraballo MA, Rötting TS, Verónica S (2010) Implementation of an MgO-based metal removal step in the passive treatment system of Shilbottle, UK: column experiments. J Hazard Mater 181:923–930
Caraballo MA, Macías F, Rötting TS, Nieto JM, Ayora C (2011) Long term remediation of highly polluted acid mine drainage: a sustainable approach to restore the environmental quality of the Odiel river basin. Environ Pollut 159:3613–3619
Caraballo MA, Serna A, Macías F, Pérez-López R, Ruiz-Cánovas C, Richter P, Becerra-Herrera M (2018) Uncertainty in the measurement of toxic metals mobility in mining/mineral wastes by standardized BCR®SEP. J Hazard Mater 360:587–593
CEAEQ (2012) Protocole de lixiviation pour les espèces inorganiques, MA. 100. http://www.ceaeq.gouv.qc.ca/methodes/pdf/MA100Lixcom11.pdf (last access October 12, 2018)
CEAEQ (2013) Détermination du carbone et du soufre : méthode par combustion et dosage par spectrophotométrie infrarouge, MA. 310 – CS 1.0, Rév. 3, Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs du Québec, 2013, 8 p. http://www.ceaeq.gouv.qc.ca/methodes/pdf/MA310CS10.pdf (last access October 12, 2018)
CEAEQ (Centre d’Expertise en Analyse Environnementale du Québec) (2003) Détermination de la matière organique par incinération : méthode de perte de feu (PAF), MA. 1010 – PAF 1.0, Ministère de l'Environnement du Québec, 2003, 9 p. http://collections.banq.qc.ca/ark:/52327/bs35531 (last access October 12, 2018)
CEAEQ (2017) Détermination des solides totaux et des solides totaux volatils : méthode gravimétrique, MA. 100 – S.T. 1.1, Rév. 5, Centre d’expertise en analyse environnementale du Québec, 2017, 13 p. http://www.ceaeq.gouv.qc.ca/methodes/pdf/MA100ST11.pdf (last access October 12, 2018)
CEN/TS 14429 (2005) Characterization of waste – Leaching behaviour tests – Influence of pH on leaching with initial Acid/base addition. Comité Européen de Normalisation, Brussels, Belgium
Couvidat J, Chatain V, Bouzahzah H, Benzaazoua M (2018) Characterization of how contaminants arise in a dredged marine sediment and analysis of the effect of natural weathering. Sci Total Environ 624:323–332
Favas PJC, Sarkar SK, Rakshit D, Venkatachalam P, Prasad MNV (2016) Acid Mine Drainages from Abandoned Mines: Hydrochemistry, Environmental Impact, Resource Recovery, and Prevention of Pollution. Environmental Materials and Waste: Resource Recovery and Pollution Prevention, Elsevier Inc
Fernández-Ondoño E, Bacchetta G, Lallena AM, Francisco BN, Ortiz I, Jiménez MN (2017) Use of BCR sequential extraction procedures for soils and plant metal transfer predictions in contaminated mine tailings in Sardinia. J Geochem Explor 172:133–141
Genty T, Neculita CM, Bussière B, Zagury, GJ (2012a) Environmental behaviour of sulphate-reducing passive bioreactor mixture. In: Proc. of the 9th International Conference on Acid Rock Drainage (ICARD), Ottawa, ON, Canada, May 20–26
Genty T, Bussière B, Benzaazoua M, Zagury GJ (2012b) Capacity of wood ash filters to remove iron from acid mine drainage: assessment of retention mechanism. Mine Water Environ 31:273–286
Genty T, Bussière B, Dionne J, Neculita CM (2016) Passive biochemical treatment of ferriferous mine drainage: Lorraine mine site, Northern Quebec, Canada. In: Proc. of the International Mine Water Association (IMWA) Annual Conference: Mining meets Water - Conflicts and Solutions, Leipzig, Germany, July 11–15
Genty T, Bussière B, Benzaazoua M, Neculita CM, Zagury GJ (2017) Iron removal in highly contaminated acid mine drainage using passive biochemical reactors. Water Sci Technol 76:833–1843
Genty T, Bussière B, Benzaazoua M, Neculita CM, Zagury GJ (2018) Changes in efficiency and hydraulic parameters during the passive treatment of ferriferous acid mine drainage in biochemical reactors. Mine Water Environ:1–10
Johnson DB, Hallberg KB (2005) Biogeochemistry of the compost bioreactor components of a composite acid mine drainage passive remediation system. Sci Total Environ 338:81–93
Jong T, Parry D (2005) Evaluation of the stability of arsenic immobilized by microbial sulfate reduction using TCLP extractions and long-term leaching techniques. Chemosphere 60:254–265
Kim JY, Kim KW, Ahn JS, Ko I, Lee CH (2005) Investigation risk assessment modeling of As and other heavy metals contamination around five abandoned metal mines in Korea. Environ Geochem Health 27(2):193–203
Kousi P, Remoundaki E, Hatzikioseyian A, Korkovelou V, Tsezos M (2018) Fractionation and leachability of Fe, Zn, Cu and Ni in the sludge from a sulphate-reducing bioreactor treating metal-bearing wastewater. Environ Sci Pollut Res 25(36):35883–35894
Lee W, Lee S, Yun S, Lee P, Hwang YS, Kim S (2016) A novel method of utilizing permeable reactive kiddle (PRK) for the remediation of acid mine drainage. J Hazard Mater 301:332–341
Liu F, Qiao X, Zhou L, Zhang J (2018) Migration and fate of acid mine drainage pollutants in calcareous soil. Int J Environ Res Public Health 15(8):1759
Macías F, Caraballo MA, Nieto JM (2012a) Environmental assessment and management of metal-rich wastes generated in acid mine drainage passive remediation systems. J Hazard Mater 229-230:107–114
Macías F, Caraballo MA, Rötting TS, Péréz-López R, Nieto JM, Ayora C (2012b) From highly polluted Zn-rich acid mine drainage to non-metallic waters: implementation of multi-step alkaline treatment system to remediate metal pollution. Sci Total Environ 433:323–330
Macías F, Pérez-López R, Caraballo MA, Canovas RC, Nieto JM (2017a) Management strategies and valorization for waste sludge from active treatment of extremely metal-polluted acid mine drainage: a contribution for sustainable mining. J Clean Prod 141:1057–1066
Macías F, Pérez-López R, Caraballo MA, Sarmiento AM, Cánovas CR, Nieto JM, Olías M, Ayora C (2017b) A geochemical approach to the restoration plans for the Odiel River basin (SW Spain), a watershed deeply polluted by acid mine drainage. Environ Sci Pollut Res 24(5):4506–4516
Martínez NM, Basallote MD, Meyer A, Cánovas CR, Macías F, Schneider P (2019) Life cycle assessment of a passive remediation system for acid mine drainage: towards more sustainable mining activity. J Clean Prod 211:1100–1111
Methods for the Chemical Analysis of Water and Wastes (MCAWW) (1978). EPA/600/4-79/020 http://www.caslab.com/EPA-Methods/PDF/EPA-Method-3762.pdf (last access October 12, 2018)
Ministère du Développement Durable, de l’Environnement et Lutte Contre les Changements Climatiques (MDDELCC) (2013) Critère de qualité de l’eau de surface. Direction de suivi de l’état de l’environnement. Bibliothèque et Archives Nationales du Québec, QC, Canada, 510p
Ministry of Justice (MOJ) (2002) Metal and Diamond Mining Effluent regulations (MDMER). Fisheries act, SOR/2002-222. http://laws-lois.justice.gc.ca/PDF/SOR-2002-222.pdf (last access October 12, 2018)
Neculita CM, Zagury GJ, Bussière B (2008) Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: II. Metal removal mechanisms and potential mobility. Appl Geochem 23:3545–3560
Nordstrom K, Blowes DW, Ptacek CJ (2015) Hydrogeochemistry and microbiology of mine drainage: an update. Appl Geochem 57:3–16
Park I, Tabelin CB, Magaribuchi K, Seno K, Ito M, Hiroyoshi N (2018) Suppression of the release of arsenic from arsenopyrite by carrier-microencapsulation using Ti-catechol complex. J Hazard Mater 344:322–332
Pinto PX, Al-Abed SR, Holder C, Reisman DJ (2014) Evaluation of metal partitioning and mobility in a sulfidic mine tailing pile under oxic and anoxic conditions. J Environ Manag 140:135–144
Rakotonimaro TV, Neculita CM, Bussière B, Zagury GJ (2016) Effectiveness of various dispersed alkaline substrates for the pre-treatment of ferriferous acid mine. Appl Geochem 73:13–23
Rakotonimaro TV, Neculita CM, Bussière B, Zagury GJ (2017a) Comparative column testing of three reactive mixtures for the bio-chemical treatment of iron-rich acid mine drainage. Miner Eng 111:79–89
Rakotonimaro TV, Neculita CM, Bussière B, Benzaazoua M, Zagury GJ (2017b) Recovery and reuse of sludge from active and passive treatment of mine drainage-impacted waters: a review. Environ Sci Pollut Res 24(1):73–91
Rakotonimaro TV, Neculita CM, Bussière B, Genty T, Zagury GJ (2018) Performance assessment of laboratory and field-scale multi-step passive treatment of iron-rich acid mine drainage for design improvement. Environ Sci Pollut Res 25(18):17575–17589
Razzouki B, Hajjaji S, Azzaoui K, Errich A, Lamhamdi A, Berrabah M, Elansari LL (2015) Physicochemical study of arsenic removal using iron hydroxide. J Mater Environ Sci 6(5):1444–1450
Rötting TS, Caraballo MA, Serrano JA, Ayora C, Carrera J (2008) Field application of calcite Dispersed Alkaline Substrate (calcite-DAS) for passive treatment of acid mine drainage with high Al and metal concentrations. Appl Geochem 23(6):1660–1674
Simonton S, Dimsha M, Thomson B, Barton LL, Cathey G (2000) Long-term stability of metals immobilized by microbial reduction. In: Proc. of the 2000 Conference on Hazardous Waste Research: Environmental Challenges and Solutions to Resource Development, Production and Use, Southeast Denver, CO, pp 394–403
Skousen J, Zipper CE, Rose A, Ziemkiewicz PF, Nairn R, Mcdonald LM, Kleinmann RL (2017) Review of passive systems for acid mine drainage treatment. Mine Water Environ 36(1):133–153
Stumm W, Morgan JJ (1996) Aquatic chemistry, chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York, USA, p 1022
Theng BKG, Yuan GD (2008) Nanoparticles in the soil environment. Elements 4:395–399
Tsiridis V, Samaras P, Kungolos A, Sakellaropoulos GP (2006) Application of leaching tests for toxicity evaluation of coal fly ash. J Env Toxicol 21(4):409–416
USEPA (2012) Method 1313: Liquid-solid partitioning as a function of extract pH using a parallel batch extraction procedure. https://www.epa.gov/sites/production/files/2015-12/documents/1313.pdf (last access October 12, 2018)
USEPA (2016) National Recommended Water Quality Criteria. https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (last access October 12, 2018)
USEPA (2017) Land disposal restrictions, rules and regulations. https://www.epa.gov/hw/land-disposal-restrictions-hazardous-waste (last access October 12, 2018)
USEPA (US Environmental Protection Agency) (1992) Method 1311: toxicity characteristic leaching procedure, SW-846: Test methods for evaluating solid waste - Physical/Chemical Methods. Washington, D.C. https://www.epa.gov/sites/production/files/2015-12/documents/1311.pdf (last access October 12, 2018)
Vadapalli K, Klink M, Etchebers O, Petrik L, Gitari W, White R, Key D, Iwuoha E (2008) Neutralization of acid mine drainage using fly ash, and strength development of the resulting solid residues. South African J Sei 104:317–322
Van Herreweghe S, Swennen R, Cappuyns V, Vandecasteele C (2002) Chemical associations of heavy metals and metalloids in contaminated soils near former ore treatment plants: a differentiated approach with emphasis on pHstat-leaching. J Geochem Explor 76(2):113–138
Funding
This study was funded by the NSERC (Natural Sciences and Engineering Research Council of Canada), Canada Research Chairs Program, the Fonds de Recherche du Québec, Nature et Technologies (FRQNT, Québec’s Research Funds, Nature and Technologies) and the industrial partners of the RIME UQAT-Polytechnique Montreal, including Agnico Eagle, Canadian Malartic Mine, Iamgold, Raglan Mine-Glencore, Rio Tinto, and Goldcorp.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Jouini, M., Rakotonimaro, T.V., Neculita, C.M. et al. Stability of metal-rich residues from laboratory multi-step treatment system for ferriferous acid mine drainage . Environ Sci Pollut Res 26, 35588–35601 (2019). https://doi.org/10.1007/s11356-019-04608-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04608-1