Abstract
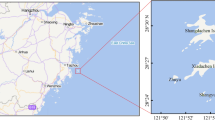
The trace elements concentration in the fish estimates the contamination degree in the aquatic environment. These toxic trace elements are transported into the human through the consumption of polluted fish. This study estimated the effect of Cd, Hg, Pb, As, and Al on tilapia species and catfish Clarias gariepinus (six for each species of fish) inhabiting Burullus Lake—Egypt 30° 33′–31° 08′ E and 30° 22′–31° 35′ N. The highest Pb concentrations recorded in the muscle of C. gariepinus 2.29 ± 0.29 μg/g while S. galilaeus was estimated the lowest Hg concentration of 0.54 ± 0.02 μg/g which indicated the presence of contaminants exceeded the limits permitted by FAO/WHO and EC. The maximum mean carbohydrate, lipid, and protein recorded in O. niloticus 18.66, 16.33, and 58.16 mg/g, respectively; moisture in O. aureus 67.33%; and ash 16.41% in O. niloticus. The lowest amount of carbohydrate was recorded in the T. zillii 14.1 mg/g, lipid, and ash in C. gariepinus 11.65 mg/g and 3.375%, respectively. Protein and moisture in the S. galilaeus were 53.75 mg/g and 60.75%, respectively. The results recorded a marked insignificant (P > 0.05) decrease in CAT, GR, and GPx activity in O. niloticus. GSH and SOD activity was an insignificant (P > 0.05) decrease in C. gariepinus. The results concluded that the trace elements concentrations exceed the maximum permissible limits recommended in fish samples set by Egypt, FAO, WHO, and EC. The estimated weekly intake of all elements through consumption of studied fish species inhabiting Burullus Lake by a child (15 kg) in Egypt is well above the PTWI recommended by FAO/WHO, whereas it is well below the PTWI for human consumption by young people (40 kg) and adult person (70 kg), at least in respect of residual levels of studied elements excluding Cd and Hg. Thus, for consumer protection, these fish species are unsafe and have hazardous effects for children, and about youth and adult consumption, caution must be taken to consider individuals eating significant amounts of fish.



Similar content being viewed by others
Change history
17 August 2020
The correct images of figs. 2 and 3 are presented in this paper.
References
Abdelazim AM, Saadeldin IM, Swelum AA, Afifi MM, Alkaladi A (2018) Oxidative stress in the muscles of the fish Nile tilapia caused by zinc oxide nanoparticles and its modulation by vitamins C and E. Oxidative Med Cell Longev:12–19. https://doi.org/10.1155/2018/6926712
Abdel-Mohsien HS, Mahmoud MAM (2015) Accumulation of some trace elements in Oreochromis niloticus from the Nile in Egypt: potential hazards to fish and consumers. J Environ Prot 6:1003–1013. https://doi.org/10.4236/jep.2015.69089
Abei H (1984) Determination of malondialdehyde. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Albering H, Rila J, Moonen E, Hoogewerff J, Kleinjans J (1999) Human health assessment in relation to environmental pollution in two artificial freshwater lakes in the Netherlands. Environ Health Perspect 107:27–35
AOAC (Association of Official Analytical Methods) (2002) Official methods of analysis, 16th edn. Arlington, Virginia
Arantes FP, Savassi LA, Santos HB, Gomes MVT, Bazzoli N (2016) Bioaccumulation of mercury, cadmium, zinc, chromium, and lead in muscle, liver, and spleen tissues of a large commercially valuable catfish species from Brazil An Acad. Bras Ciências 88:137–147. https://doi.org/10.1590/0001-3765201620140434
Arora M, Kiran B, Rani S, Rani A, Kaur B, Mittal N (2008) Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem 11:811–815. https://doi.org/10.1016/j.foodchem.2008.04.049
ATSDR (Agency for Toxic Substances and Disease Registry) (2007) Toxicological profile for lead. Public Health Service, U.S. Department of Health and Human Services, Atlanta
Authman MMN (2011) Environmental and experimental studies of aluminum toxicity on the liver of Oreochromis niloticus (Linnaeus, 1757) fish. Life Sci J 8:764–776
Authman MMN, Ibrahim SA, El-Kasheif MA, Gaber HS (2013) Heavy metals pollution and their effects on gills and liver of the Nile catfish inhabiting El-Rahawy drain, Egypt. Glob Vet 10(2):103–115
Beutler E, Duron O, Kelly MB (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Birungi Z, Masola B, Zaranyika MF, Naigaga I, Marshall B (2007) Active biomonitoring of trace heavy metals using fish (Oreochromis niloticus) as bioindicator species. The case of Nakivubo wetland along Lake Victoria. Phys Chem Earth 32:1350–1358
Dempson JB, Schwarz CJ, Shears M, Furey G (2004) Comparative proximate body composition of Atlantic salmon with emphasis on parr from fluvial and lacustrine habitats. J Fish Biol 64:1257–1271
Dimari GA, Abdulrahman JC, Garba ST (2008) Metals concentrations in tissues of Tilapia Galli, Clarias lazera and Osteoglossidae caught from Alau Dam, Maiduguri, Borno State, Nigeria. Am J Environ Sci 4:373–379. https://doi.org/10.3844/ajessp.2008.373.37910.3844/ajessp
Donohue JM, Abernathy CO (1999) Exposure to inorganic arsenic from fish and shellfish. In: Chappell WR, Abernathy CO, Calderon RL (eds) Arsenic exposure and health effects. Elsevier, Oxford, pp 89–98
EC (2006) Commission regulation no.1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (text with EEA relevance). Off J Eur Communities L364:5–24
EC (2008) Commission regulation no.629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off J Eur Communities L173:6–9
EC (2011) Commission regulation no.420/2011 of 29 April 2011 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off J Eur Communities L111:3–6
El-Batrawy OA, El-Gammal I, Mohamadein LI, Darwish DH, El-Moselhy Kh M (2018) Impact assessment of some heavy metals on tilapia fish, Oreochromis niloticus, in Burullus Lake, Egypt. JoBAZ 79:13. https://doi.org/10.1186/s41936-018-0028-4
El-Sappah AH, Shawky AS, Sayed-Ahmad MS, Youssef MAH (2012) Nile tilapia as bioindicator to estimate the contamination of water using SDS-PAGE and RAPD-PCR techniques. Egypt J Genet Cytol 41:209–227
EMI (2012) Egyptian Ministry of Irrigation, the amount of agricultural drainage water which entered the Lake Burullus during 2010. Organization of Mechanic and Electricity, Central Administration of the Central Delta stations, Kafr El- Sheikh
EOSQC (1993) Egyptian Organization for Standardization and Quality Control, maximum residue limits for heavy metals in food. Ministry of Industry No. 2360/1993, 5.
EPA (Environmental Protection Agency) (2002) Risk assessment: technical background information. RBG table
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539. https://doi.org/10.2174/1568026013394831
FAO (2003) Fisheries and aquaculture topics. Quality and safety of fish and fish products. Topical fact Sheets. In: FAO Fisheries and Aquaculture department
FAO (2010) The international fish trade and world fisheries, https://www.fao.org/fileadmin/user_upload/newsroom/docs/fact_sheet_fish_trade_en.pd. Accessed 1 Apr 2010
FAO/WHO (1992) Food Monitoring and Assessment Programme, WHO, Geneva 5, UNEP, Nairobi. 52. Report of the Third Meeting of the GEMS/Food
FAO/WHO (1993) Evaluation of certain food additives and contaminants (Fourth-First Report of Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series No. 837, WHO, Geneva
FAO/WHO (1999) Expert Committee on Food Additives. Summary and conclusion, 53rd meeting, Rome, 1-10 June.
FAO/WHO (2004) Summary of evaluations performed by the joint FAO/WHO Expert Committee on Food Additives (JECFA 1956-2003), (First through Sixty First Meetings). ILSI Press International Life Sciences Institute
Fazio F, Piccione G, Tribulato K, Ferrantelli V, Giangrosso G, Arfuso F, Faggio C (2014) Bioaccumulation of heavy metals in blood and tissue of striped mullet in two Italian Lakes. J Aquat Anim Health 26(4):278–284. https://doi.org/10.1080/08997659.2014.938872
Flora SJS, Mittal M, Mehta A (2008) Heavy metal-induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128:501–523
Fozia A, Muhammad AZ, Muhammad A, Zafar MK (2008) Effect of chromium on growth attributes in sunflower (Helianthus annuus L.). J Environ Sci 20(12):1475–1480. https://doi.org/10.1016/s1001-0742(08)62552-8
GAFRD (2006) General Authority for Fishery Resources Development. Year-book of fishery statistics in Egypt (1990-2005), Cairo, Egypt.
GAFRD (2015) General authority for fish resources development, yearbook of fishery statistics Cairo-Egypt
Garcia-Medina S, Razo-Estrada AC, Gomez-Olivan LM, Amaya-Chavez A, Madrigal-Bujaidar E, Galar-Martínez M (2010) Aluminium-induced oxidative stress in lymphocytes of common carp (Cyprinus carpio). Fish Physiol Biochem 36:875–882. https://doi.org/10.1007/s10695-009-9363-1
Goldberg DM, Spooner RJ (1983) In: Bergmeyen HV (ed) Methods of enzymatic analysis, vol 3, 3rd edn. Verlog Chemie, Deerfield beach, pp 258–265
Govind P, Madhuri S (2014) Heavy metals causing toxicity in animals and fishes. Res. J. Ani, Vet and Fish Sci. 2(2):17–23
Gul S, Hasan Z, Khan GN, Gul S, Attaullah (2017) Proximate body composition of five commercial fish species of family Cyprinida commonly consumed in Swat Khyber Pakhtunkhwa Pakistan. J Entomol Zool Stud 5(3):1255–1257
Henry RJ, Cannon DC, Winkelman W (1974) Clinical chemistry principles and techniques, vol 1629, 11th edn. Harper and Row Publishers, N Y, pp 528–538
IARC, International Agency for Research on Cancer (1993) Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. In: International agency for research on cancer monographs on the evaluation of carcinogenic risks to humans, vol 58. IARC(International Arctic Research Center) Sci Publ, Lyon, pp 119–237
Kaoud HA, El-Dahshan AR (2010) Bioaccumulation and histopathological alterations of the heavy metals in Oreochromis niloticus Fish. Nat Sci 8:147–156
Kemp A, Adrienne JM, Van K, Hejningen (1954) A colorimetric method for the determination of glycogen in tissues. The Biochemical Journal 56:640–648
Koch I, Reimer KJ, Beach A, Cullen WR, Gosden A, Lai VWM (2001) Arsenic speciation in fresh-water fish and bivalves. Elsevier Science Ltd. Heal. and Enviro. Res, Oxford, pp 115–123
Kris-Etherton P, Harris W, Appel L (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757
Laitinen M, Valtonen T (1995) Cardiovascular, ventilatory and haematological responses of brown trout (Salmo trutta L.) to the combined effects of acidity and aluminum in humic water at winter temperatures. Aquat Toxicol 31:99–112
Larry B, Lobring, Billy B P (1993) Inorganic of mercury in tissues by cod vapor atomic absorption spectrometery, environmental monitoring system laboratory office of research and development. U.S Environmental protection agency cincinmal I, OHIO 45268
Lilly TT, Immaculate JK, Jamila P (2017) Macro and micronutrients of selected marine fishes in Tuticorin, South East coast of India. Int J Food Res 24(1):191–201 http://www.ifrj.upm.edu.my. Accessed Feb 2010
Lowry OH, Rosenbrough NJ, Farr RL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mensoor M, Said A (2018) Determination of heavy metals in freshwater fishes of the Tigris River in Baghdad Montazer. Fishes 3:23. https://doi.org/10.3390/fishes3020023
Mohamed FAS, Gad NSH (2005) Distribution of some heavy metals in tissues of (Oreochroms siloticus, Tilapia zilli and Clarias lazera) from Abu za’baal Lakes and their impacts on some biochemical parameters and on the histological structures of some organs. Egypt J Aquau Biol 9(1):41–50
Mohamed FA, Gad NS (2008) Environmental pollution induced biochemical changes in tissues of Tilapia zillii, Solea vulgaris and Mugil capito from Lake Qarun, Egypt. Glob Vet 2(6):327–336
Monteiro DA, Rantin FT, Kalinin AL (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxa, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19:105–123
Mylroie AA, Umbles C, Kyle J (1984) Effects of dietary copper supplementation on erythrocyte superoxide dismutase activity, ceruloplasmin and related parameters in rats ingesting lead acetate. In: Hemphill (ed) Trace substances in environ health, vol 18. University of Missouri Press, Columbia, pp 497–504. https://doi.org/10.1016/j.jfca.2013.08.007
Nishikimi M, Roa NA, Yogi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854. https://doi.org/10.1016/S0006-291X(72)80218-3
NNDSR (2010) National Nutrient Database for Standard Reference. USDA, Washington, DC Release No. 18
Noor N, Zutshi B (2016) Bioaccumulation of trace metals in tissues of Rohu fish for environmental risk assessment. J Wat Res Prot 8:472–481
Olabanji IO, Oluyemi EA (2014) Preliminary assessment of heavy metal pollution of Opa Reservoir. Ile- Ife, Southwest Nigeria using Mormyrus Rumea (Tilapia zillii). IFE J Sci 16:1
Olagunju A, Muhammad A, Mada SB, Mohammed A, Mohammed HM, Mahmoud KT (2012) Nutrient composition of Tilapia zilli, Hemi-synodontis membranacea, Clupea harengus, and Scomber scombrus locally consumed in Africa. World J Life Sci Med Res 2:16–19
Oliveira Ribeiro CA, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aqua Toxicol 74:53–69
Olojo EAA, Olurin KB, mbaka G, Oluwemimo AD (2005) Histopathology of the gill and liver tissues of the African catfish (Clarias gariepinus) exposed to lead. Afr J Biotechnol 4(1):117–122
Orun I, Talas ZS, Ozdemir I, Alkan A, Erdogan K (2008) Antioxidative role of selenium on some tissues of (Cd2+, Cr3+)-induced rainbow trout. Ecotoxicol Environ Saf 71:71–75
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxidase. J Lab Clin Med 70:158–165
Qin D, Jiang H, Bai S, Tang S, Mou Z (2015) Determination of 28 trace elements in three farmed cyprinid fish species from northeast China. Food Control 50:1–8. https://doi.org/10.1016/j.foodcont.2014.08.016
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang bay, Taihu Lake, China. Toxicol Rep 5:288–295. https://doi.org/10.1016/j.toxrep.2018.01.007
Roberts JR (1999) Metal toxicity in children. In: Training Manual on Pediatric Environmental Health. Children’s Environmental Health, Emeryville
Romeo M, Bennani N, Gnassia-Barelli M, Lafaurie M, Girard JP (2000) Cadmium and copper display different responses towards oxidative stress in the kidney of the sea bass Dicentrarchus labrax. AquaticToxicology 48:185–194
Saglam D, Atli G, Dogan Z, BaysoyE GC, Eroglu A, Canli M (2014) Response of the antioxidant system of freshwater fish (Oreochromis niloticus) exposed to metals (Cd, Cu) in differing hardness. Turk Jof Fish Aqu Sci 14:43–52. https://doi.org/10.4194/1303-2712-v14_1_06
Salas J, Font I, Canals J, Guinovart L, Sospedrav C, Martin-Hennenberg C (1985) Consumption, nutritional habits and nutritional status of the population from Reus. II. Age and sex distribution of the consumption of meat, fish, eggs and Pulses. Med. Clin. 84:423–427
Salazar-Flores J, Torres-Jasso JH, Rojas-Bravo D, Reyna-Villela ZM, Torres-Sánchez E (2019) Effects of mercury, lead, arsenic and zinc to human renal oxidative stress and functions: a review. J Heavy Met Toxic 4:–2. https://doi.org/10.21767/2473-6457.10027
Sanfeliu C, Sebastia J, Kim U (2001) Methylmercury neurotoxicity in cultures of human neurons, astrocytes, neuroblastoma cells. Neurotoxicology. 22:317–327. https://doi.org/10.1016/S0161-813X(01)00015-8
Sarah R, Tabassum B, Idrees N, Hashem A, Abd-Allah EF (2019) Bioaccumulation of heavy metals in Channa punctatus (Bloch) in river Ramganga (U.P.), India. Saudi Journal of Biological Sciences 26:979–984. https://doi.org/10.1016/j.sjbs.2019.02.009
Schmidt Nielson B (1975) Osmoregulation: effect of salinity and heavy metal. Fed. Proc. 33:2137–2146
Shah AQ, Kazi TG, Arain MB, Jamali MK, Afridi HI, Jalbani N, Baig JA, Kandhro GA (2009) Accumulation of arsenic in different freshwater fish species – potential contribution to high arsenic intakes. Food Chem 112:520–524
Sidwell VD, Stallings BR, Knobble M (1970) The fish protein concentration. Nutritional quality and using fish in foods. J FoodTechnol 14:40–46
Sinclair ARE, Duncan P (1972) Indices of condition in tropical ruminants. East Africa Wild J 10:143–149
Sobha K, Poornima A, Harini P, Veeraiah KA (2007) Study on biochemical changes in the freshwater fish Catla catla (Hamilton), exposed to the heavy metal toxicant cadmium chloride. Kathmandu Univ. J Sci Eng Technol 1(14):1–11
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metals ions. Free Radic Biol Med 2:321–336
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18(3):149–175
Wätjen W, Beyersmann D (2004) Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals 17(1):65–78
WHO (2006) Evaluation of certain food contaminants. Sixty-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series, No. 930
Yacoub AM, Gad NS (2012) Alterations in muscles of Oreochromis niloticus from the River Nile in Upper Egypt. Inter J of Environ Sci and Eng 3:1–10
Yahya AN, Mohamed SK, Mohamed AG (2018) Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Acc J of Toxicol 3(1):555603. https://doi.org/10.19080/OAJT.2018.03.555603
Zhang W, Wang WX (2012) Large-scale spatial and interspecies differences in trace elements and stable isotopes in marine wild fish from Chinese waters. J Hazard Mater 215:65–74. https://doi.org/10.1016/j.jhazmat.2012.02.032
Funding
The research was supported by the National Institute of Oceanography and Fisheries (NIOF), Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Capture and necropsy techniques were performed according to guidelines for ethical conduct in the care and use of non-human animals for research that was developed by the committee of the American Psychological Association for animal research and ethics in 2010–2011.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Cd, Hg, Pb, As, and Al accumulation in the Burullus Lake—Egypt elicited a physiological response in tilapia species and Clarias gariepinus.
• Trace element concentrations exceed the maximum permissible limits recommended in fish samples set by Egypt, FAO, WHO, and EC.
• Proximate body composition and antioxidant enzyme activities showed altered values.
• The estimated weekly intake of elements for a child (15 kg) consuming fish in Egypt was above the PTWI recommended by FAO/WHO for all elements.
• The estimated weekly intake of elements for (40 kg) young people and (70 kg) adult person consuming fish in Egypt was well below the PTWI recommended by FAO/WHO except for Cd and Hg.
• These fish species are unsafe and have hazardous effects for children.
• The consumption of these species inhabiting the Burullus Lake for youth and adult people is safe and not toxic for human health except for Cd and Hg.
• Caution must be taken to consider individuals eating significant amounts of fish.
Rights and permissions
About this article
Cite this article
Abdel-Kader, H.H., Mourad, M.H. Trace elements exposure influences proximate body composition and antioxidant enzyme activities of the species tilapia and catfish in Burullus Lake—Egypt: human risk assessment for the consumers. Environ Sci Pollut Res 27, 43670–43681 (2020). https://doi.org/10.1007/s11356-020-10207-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10207-2