Abstract
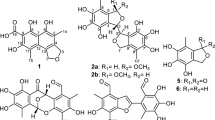
Sesbagrandiflorains A (1) and B (2), isolated from the stem bark of the Indonesian fabaceous plant Sesbania grandiflora, were reported to be 6-methoxy-2-(2´,3´-dihydroxy-5´-methoxyphenyl)-1-benzofuran-3-carbaldehyde and 6-hydroxy-2-(2´,3´-dihydroxy-5´-methoxyphenyl)-1-benzofuran-3-carbaldehyde, respectively. However, based on reevaluation of their 1D and 2D NMR data, the chemical structures of 1 and 2 have been revised to 4-hydroxy-2-(4´-hydroxy-2´-methoxyphenyl)-6-methoxybenzofuran-3-carbaldehyde and 4-hydroxy-2-(4´-hydroxy-2´-hydroxyphenyl)-6-methoxybenzofuran-3-carbaldehyde, respectively. In addition, seven new derivatives of 1 have been synthesized from the natural product in good yields (65 − 93%). The chemical structures of the synthetic compounds—one diester (6), four ethers (7–10), one secondary amine (11), and one oxime (12)—were confirmed by MS and NMR analysis. Compound 6 exhibited moderate antibacterial activity against the plant pathogen Rhodococcus fascians with a MIC of 0.1 mg/mL. Compounds 8 and 12 demonstrated respectable cytotoxicity against A375 melanoma cancer cells line with the relative IC50 values of 22.8 and 32.7 μM, respectively.




Similar content being viewed by others
References
Nuntawong P, Kongkatitham V, Likhitwitayawuid K, Mekboonsonglarp W, Sukrong S, Tanasupawat S, Sritularak B (2019) New 2-arylbenzofurans from the root bark of Artocarpus gomezianus and their alpha-glucosidase inhibitory activity. Nat Prod Res 33:1436–1441
Han SJ, Ryu SN, Kang SS (2004) A new 2-arylbenzofuran with antioxidant activity from the black colored rice (Oryza sativa L.) bran. Chem Pharm Bull 52:1365–1366
Kyekyeku JO, Kusari S, Adosraku RK, Zuhlke S, Spiteller M (2016) Prenylated 2-arylbenzofuran derivatives with potent antioxidant properties from Chlorophora regia (Moraceae). Fitoterapia 108:41–47
Paudel P, Seong SH, Wagle A, Min BS, Jung HA, Choi JS (2020) Antioxidant and anti-browning property of 2-arylbenzofuran derivatives from Morus alba Linn root bark. Food Chem 309:125739
Tan YX, Gong T, Liu C, Chen RY, Yu DQ (2010) Five new 2-arylbenzofuran derivatives from Morus wittiorum. Chem Pharm Bull 58:579–581
Hu X, Wang M, Yan GR, Yu MH, Wang HY, Hou AJ (2012) 2-Arylbenzofuran and tyrosinase inhibitory constituents of Morus notabilis. J Asian Nat Prod Res 14:1103–1108
Katsanou ES, Halabalaki M, Aligiannis N, Mitakou S, Skaltsounis AL, Alexi X, Pratsinis H, Alexis MN (2007) Cytotoxic effects of 2-arylbenzofuran phytoestrogens on human cancer cells: modulation by adrenal and gonadal steroids. J Steroid Biochem Mol Biol 104:228–236
Juliawaty LD, Sahidin HEH, Achmad SA, Syah YM, Latip J, Said IM (2009) A 2-arylbenzofuran derivative from Hopea mengarawan. Nat Prod Commun 4:947–950
Mansoor TA, Borralho PM, Luo X, Mulhovo S, Rodrigues CM, Ferreira MJ (2013) Apoptosis inducing activity of benzophenanthridine-type alkaloids and 2-arylbenzofuran neolignans in HCT116 colon carcinoma cells. Phytomedicine 20:923–929
Ni G, Zhang QJ, Zheng ZF, Chen RY, Yu DQ (2009) 2-Arylbenzofuran Derivatives from Morus cathayana. J Nat Prod 72:966–968
Yang Y, Gong T, Liu C, Chen RY (2010) Four new 2-arylbenzofuran derivatives from leaves of Morus alba L. Chem Pharm Bull 58:257–260
Rizzo S, Tarozzi A, Bartolini M, Da Costa G, Bisi A, Gobbi S, Belluti F, Ligresti A, Allara M, Monti JP, Andrisano V, Di Marzo V, Hrelia P, Rampa A (2012) 2-Arylbenzofuran-based molecules as multipotent Alzheimer's disease modifying agents. Eur J Med Chem 58:519–532
Nomura T, Fukai T (1981) Mulberrofuran B, a new isoprenoid 2-arylbenzofuran from the root bark of the cultivated mulberry tree. Planta Med 42:197–199
Shi YQ, Nomura T, Fukai T (2007) A new 2-arylbenzofuran from the root bark of Chinese Morus cathayana. Fitoterapia 78:617–618
Basnet P, Kadota S, Terashima S, ShimizuM NT (1993) Two new 2-arylbenzofuran derivatives from hypoglycemic activity-bearing fractions of Morus insignis. Chem Pharm Bull 41:1238–1243
Hu X, Wu JW, Wang M, Yu MH, Zhao QS, Wang HY, Hou AJ (2012) 2-Arylbenzofuran, flavonoid, and tyrosinase inhibitory constituents of Morus yunnanensis. J Nat Prod 75:82–87
Rios-Motta J, Avella E (2010) 2-Arylbenzofuran neolignans from the bark of Nectandra purpurascens (Lauraceae). Nat Prod Commun 5:1063–1066
Kanchanapoom T, Suga K, Kasai R, Yamasaki K, Kamel MS, Mohamed MH (2002) Stilbene and 2-arylbenzofuran glucosides from the rhizomes of Schoenocaulon officinale. Chem Pharm Bull 50:863–865
Yenesew A, Midiwo JO, Guchu SM, Heydenreich M, Peter MG (2002) Three isoflav-3-enes and a 2-arylbenzofuran from the root bark of Erythrina burttii. Phytochemistry 59:337–341
Luo G, Yang Y, Zhou M, Ye Q, Liu Y, Gu J, Zhang G, Luo Y (2014) Novel 2-arylbenzofuran dimers and polyisoprenylated flavanones from Sophora tonkinensis. Fitoterapia 99:21–27
Kraft C, Jenett-Siems K, Siems K, Solis PN, Gupta MP, Bienzle U, Eich E (2001) Andinermals A-C, antiplasmodial constituents from Andira inermis. Phytochemistry 58:769–774
Tanaka H, Hirata M, Etoh H, Sako M, Sato M, Murata J, Murata H, Darnaedi D, Fukai T (2004) Six new constituents from the roots of Erythrina variegata. Chem Biodivers 1:1101–1108
Wang W, Zhao YY, Wang B, Liang H, Tu GZ, Chen HB (2007) Two new arylbenzofurans from the roots of Hedysarum multijugum. J Asian Nat Prod Res 9:19–22
Wang S, Zhang G, Guan J, Zhu L, Chen L, Pan C, Li P, Li L (2009) A new arylbenzofuran from the aerial parts of alfalfa. J Nat Med 63:189–191
Halabalaki M, Alexi X, Aligiannis N, Alexis MN, Skaltsounis AL (2008) Ebenfurans IV-VIII from Onobrychis ebenoides: evidence that C-prenylation is the key determinant of the cytotoxicity of 3-formyl-2-arylbenzofurans. J Nat Prod 71:1934–1937
Halabalaki M, Aligiannis N, Papoutsi Z, Mitakou S, Moutsatsou P, Sekeris C, Skaltsounis AL (2000) Three new arylobenzofurans from Onobrychis ebenoides and evaluation of their binding affinity for the estrogen receptor. J Nat Prod 63:1672–1674
Luo G, Zhou M, Liu Y, Ye Q, Gu J, Huang T, Zhang G, Luo Y (2014) 3-Formyl-2-arylbenzofurans from the aerial parts of Itea ilicifolia. Phytochem Lett 10:19–22
Chang JY, Chang CY, Kuo CC, Chen LT, Wein YS, Kuo YH (2004) Salvinal, a novel microtubule inhibitor isolated from Salvia miltiorrhizae Bunge (Danshen), with antimitotic activity in multidrug-sensitive and -resistant human tumor cells. Mol Pharmacol 65:77–84
Noviany N, Nurhidayat A, Hadi S, Suhartati T, Aziz M, Purwitasari N, Subasman I (2018) Sesbagrandiflorain A and B: isolation of two new 2-arylbenzofurans from the stem bark of Sesbania grandiflora. Nat Prod Res 32:2558–2564
Noviany N, Samadi A, Yuliyan N, Hadi S, Aziz M, Purwitasari N, Mohamad S, Ismail NN, Gable KP, Mahmud T (2020) Structure characterization and biological activity of 2-arylbenzofurans from an Indonesian plant, Sesbania grandiflora (L.) Pers. Phytochem Lett 35:211–215
Acknowledgements
The authors thank the Directorate of Research and Community Services, Directorate General of Higher Education, The Ministry of Research, Technology and Higher Education, Republic of Indonesia for providing funds for this project through PSN Grant 2017-2019 (No. 860/UN26.21/PN/2019), PDUPT Grant 2019 (No. 856/UN26.21/PN/2019) and World Class Professor Program-Scheme B 2018 (No.123.44/D2.3/KP/2018). Work at Oregon State University was funded by the National Institute of Food and Agriculture of the U.S. Department of Agriculture under Award Number 2014-51181-22384 and the National Center for Complementary & Integrative Health of the National Institutes of Health under Award Number T32AT010131. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Agriculture and/or the National Institutes of Health. We acknowledge the support of the Oregon State University NMR Facility funded in part by the National Institutes of Health, HEI Grant 1S10OD018518, and by the M. J. Murdock Charitable Trust grant #2014162.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noviany, N., Samadi, A., Carpenter, E.L. et al. Structural revision of sesbagrandiflorains A and B, and synthesis and biological evaluation of 6-methoxy-2-arylbenzofuran derivatives. J Nat Med 75, 66–75 (2021). https://doi.org/10.1007/s11418-020-01445-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-020-01445-2