Abstract
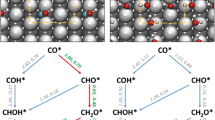
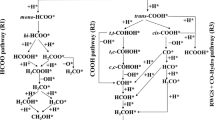
The hydrogenation of carbon dioxide (CO2) is one of important processes to effectively convert and utilize CO2, which is also regarded as the key step at the industrial methanol synthesis. Water is likely to play an important role in this process, but it still remains elusive. To systematically understand its influence, here we computationally compare the reaction mechanisms of CO2 hydrogenation over the stepped Cu(211) surface between in the absence and presence of water based on microkinetic simulations upon density functional theory (DFT) calculations. The effects of water on each hydrogenation step and the whole activity and selectivity are checked and its physical origin is discussed. It is found that the water could kinetically accelerate the hydrogenation on CO2 to COOH, promoting the reverse water gas shift reaction to produce carbon monoxide (CO). It hardly influences the CO2 hydrogenation to methanol kinetically. In addition, the too high initial partial pressure of water will thermodynamically inhibit the CO2 conversion.
Similar content being viewed by others
References
Chinchen GC, Denny PJ, Jennings JR, Spencer MS, Waugh KC. Appl Catal, 1988, 36: 1–65
Yin X, Moss JR. Coord Chem Rev, 1999, 181: 27–59
Shi C, Chan K, Yoo JS, Nørskov JK. Org Process Res Dev, 2016, 20: 1424–1430
Behrens M. J Catal, 2009, 267: 24–29
Kattel S, Ramírez PJ, Chen JG, Rodriguez JA, Liu P. Science, 2017, 357: eaan8210
Zurbel A, Kraft M, Kavurucu-Schubert S, Bertau M. Chem Ingenieur Technik, 2018, 90: 721–724
Yang Y, Mims CA, Mei DH, Peden CHF, Campbell CT. J Catal, 2013, 298: 10–17
Dietz L, Piccinin S, Maestri M. J Phys Chem C, 2015, 119: 4959–4966
Chen Y, Cheng J, Hu P, Wang H. Surf Sci, 2008, 602: 2828–2834
Garza AJ, Bell AT, Head-Gordon M. ACS Catal, 2018, 8: 1490–1499
Chinchen GC, Denny PJ, Parker DG, Spencer MS, Whan DA. Appl Catal, 1987, 30: 333–338
Hansen JB, Højlund Nielsen PE. Methanol synthesis. In: Knozinger GEH, Schuth F, Weitkamp J, Eds. Handbook of Heterogeneous Catalysis. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2008. 2920–2949
Liu XM, Lu GQ, Yan ZF, Beltramini J. Ind Eng Chem Res, 2003, 42: 6518–6530
Behrens M, Studt F, Kasatkin I, Kühl S, Hävecker M, Abild-Pedersen F, Zander S, Girgsdies F, Kurr P, Kniep BL, Tovar M, Fischer RW, Nørskov JK, Schlögl R. Science, 2012, 336: 893–897
Grabow LC, Mavrikakis M. ACS Catal, 2011, 1: 365–384
Zhao YF, Yang Y, Mims C, Peden CHF, Li J, Mei D. J Catal, 2011, 281: 199–211
Sun X, Cao X, Hu P. Sci China Chem, 2015, 58: 553–564
Burch R, Golunski SE, Spencer MS. Catal Lett, 1990, 5: 55–60
Kattel S, Yan B, Yang Y, Chen JG, Liu P. J Am Chem Soc, 2016, 138: 12440–12450
Klier K, Chatikavanij V, Herman RG, Simmons GW. J Catal, 1982, 74: 343–360
Parameswaran VR, Lee S, Wender I. Fuel Sci Tech Int, 1989, 7: 899–918
Liu G, Willcox D, Garland M, Kung HH. J Catal, 1984, 90: 139–146
He Z, Qian Q, Ma J, Meng Q, Zhou H, Song J, Liu Z, Han B. Angew Chem Int Ed, 2016, 55: 737–741
Kresse G, Furthmüller J. Phys Rev B, 1996, 54: 11169–11186
Perdew JP, Burke K, Ernzerhof M. Phys Rev Lett, 1996, 77: 3865–3868
Kresse G, Joubert D. Phys Rev B, 1999, 59: 1758–1775
Blöchl PE. Phys Rev B, 1994, 50: 17953–17979
Haynes WM. CRC Handbook of Chemistry and Physics. Lodon & New York: CRC Press, 2014
Alavi A, Hu P, Deutsch T, Silvestrelli PL, Hutter J. Phys Rev Lett, 1998, 80: 3650–3653
Michaelides A, Liu ZP, Zhang CJ, Alavi A, King DA, Hu P. J Am Chem Soc, 2003, 125: 3704–3705
Liu ZP, Hu P. J Am Chem Soc, 2003, 125: 1958–1967
Schenter GK, Mills G, Jónsson H. J Chem Phys, 1994, 101: 8964–8971
Mills G, Jónsson H, Schenter GK. Surf Sci, 1995, 324: 305–337
Henkelman G, Jónsson H. J Chem Phys, 2000, 113: 9978–9985
Cao XM, Burch R, Hardacre C, Hu P. Catal Today, 2011, 165: 71–79
Wang Z, Cao XM, Zhu J, Hu P. J Catal, 2014, 311: 469–480
Studt F, Behrens M, Kunkes EL, Thomas N, Zander S, Tarasov A, Schumann J, Frei E, Varley JB, Abild-Pedersen F, Nørskov JK, Schlögl R. ChemCatChem, 2015, 7: 1105–1111
Zhang L, Shao ZJ, Cao XM, Hu P. J Phys Chem C, 2018, 122: 20337–20350
Wang Z, Liu X, Rooney DW, Hu P. Surf Sci, 2015, 640: 181–189
Rasmussen PB, Kazuta M, Chorkendorff I. Surf Sci, 1994, 318: 267–280
Hong QJ, Liu ZP. Surf Sci, 2010, 604: 1869–1876
Yang Y, Mims CA, Disselkamp RS, Peden CHF, Campbell CT. Top Catal, 2009, 52: 1440–1447
Chen CS, Wu JH, Lai TW. J Phys Chem C, 2010, 114: 15021–15028
Studt F, Abild-Pedersen F, Varley JB, Nørskov JK. Catal Lett, 2013, 143: 71–73
Nie X, Esopi MR, Janik MJ, Asthagiri A. Angew Chem Int Ed, 2013, 52: 2459–2462
Li Z, Wang J, Qu Y, Liu H, Tang C, Miao S, Feng Z, An H, Li C. ACS Catal, 2017, 7: 8544–8548
Wang J, Li G, Li Z, Tang C, Feng Z, An H, Liu H, Liu T, Li C. Sci Adv, 2017, 3: e1701290
Gokhale AA, Dumesic JA, Mavrikakis M. J Am Chem Soc, 2008, 130: 1402–1414
Álvarez A, Borges M, Corral-Pérez JJ, Olcina JG, Hu L, Cornu D, Huang R, Stoian D, Urakawa A. Chem Phys Chem, 2017, 18: 3135–3141
Yin LL, Gong XQ. Sci China Chem, 2015, 58: 601–606
Yang M, Yuan H, Wang H, Hu P. Sci China Chem, 2018, 61: 457–467
Yuan H, Sun N, Chen J, Jin J, Wang H, Hu P. ACS Catal, 2018, 8: 9269–9279
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0208600), the National Natural Science Foundation of China (21673072, 21333003, 91845111), and Program of Shanghai Subject Chief Scientist (17XD1401400).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sun, X., Wang, P., Shao, Z. et al. A first-principles microkinetic study on the hydrogenation of carbon dioxide over Cu(211) in the presence of water. Sci. China Chem. 62, 1686–1697 (2019). https://doi.org/10.1007/s11426-019-9639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-019-9639-0