Abstract
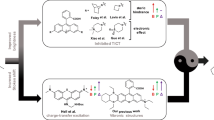
Uni-directional multi-state fluorochromic scaffolds are valuable photofunctional molecules and yet scarce. We report a general approach for their design, i.e., mechanodonor-acceptor coupling (MDAC). A photochromic molecule is a mechanodonor, due to its capability to convert photonic energy into mechanical force. Upon proper coupling, it can be used to drive a mechanochromic molecule for uni-directional multi-state fluorochromism. The embodiment of this approach is a rhodamine-dithienylethylene hydride (RDH), which has been successfully employed in super-resolution localization microscopy

Similar content being viewed by others
References
Bamfield P. Chromic Phenomena: Technological Applications of Colour Chemistry. Cambridge: RSC, 2001
Singh A, Amiji MM. Stimuli-responsive Drug Delivery Systems. Cambridge: RSC, 2018
Xi H, Zhang Z, Zhang W, Li M, Lian C, Luo Q, Tian H, Zhu WH. J Am Chem Soc, 2019, 141: 18467–18474
Uno K, Bossi ML, Irie M, Belov VN, Hell SW. J Am Chem Soc, 2019, 141: 16471–16478
Roubinet B, Weber M, Shojaei H, Bates M, Bossi ML, Belov VN, Irie M, Hell SW. J Am Chem Soc, 2017, 139: 6611–6620
Hüll K, Morstein J, Trauner D. Chem Rev, 2018, 118: 10710–10747
Fuchter MJ. J Med Chem, 2020, 63: 11436–11447
Morstein J, Dacheux MA, Norman DD, Shemet A, Donthamsetti PC, Citir M, Frank JA, Schultz C, Isacoff EY, Parrill AL, Tigyi GJ, Trauner D. J Am Chem Soc, 2020, 142: 10612–10616
Borowiak M, Küllmer F, Gegenfurtner F, Peil S, Nasufovic V, Zahler S, Thorn-Seshold O, Trauner D, Arndt HD. J Am Chem Soc, 2020, 142: 9240–9249
Cheng B, Morstein J, Ladefoged LK, Maesen JB, Schiøtt B, Sinning S, Trauner D. ACS Chem Neurosci, 2020, 11: 1231–1237
Tian T, Song Y, Wang J, Fu B, He Z, Xu X, Li A, Zhou X, Wang S, Zhou X. J Am Chem Soc, 2016, 138: 955–961
Velema WA, Szymanski W, Feringa BL. J Am Chem Soc, 2014, 136: 2178–2191
Silva APD. Molecular Logic-based Computation. Cambridge: RSC, 2012
Erbas-Cakmak S, Kolemen S, Sedgwick AC, Gunnlaugsson T, James TD, Yoon J, Akkaya EU. Chem Soc Rev, 2018, 47: 2228–2248
Feringa BL, Browne WR. Molecular Switches. Weinheim: John Wiley & Sons, 2011
Ellis GP. Chromenes. Chromanones, and Chromones. New York: John Wiley & Sons, 1977
Zheng LQ, Yang S, Lan J, Gyr L, Goubert G, Qian H, Aprahamian I, Zenobi R. J Am Chem Soc, 2019, 141: 17637–17645
Shao B, Qian H, Li Q, Aprahamian I. J Am Chem Soc, 2019, 141: 8364–8371
Yang Y, Hughes RP, Aprahamian I. J Am Chem Soc, 2014, 136: 13190–13193
Rao YL, Chen LD, Mosey NJ, Wang S. J Am Chem Soc, 2012, 134: 11026–11034
Bellotto S, Chen S, Rentero Rebollo I, Wegner HA, Heinis C. J Am Chem Soc, 2014, 136: 5880–5883
Yonekawa I, Mutoh K, Kobayashi Y, Abe J. J Am Chem Soc, 2018, 140: 1091–1097
Sajimon MC, Ramaiah D, Suresh CH, Adam W, Lewis FD, George MV. J Am Chem Soc, 2007, 129: 9439–9445
Hammerich M, Schütt C, Stähler C, Lentes P, Röhricht F, Höppner R, Herges R. J Am Chem Soc, 2016, 138: 13111–13114
Hemmer JR, Poelma SO, Treat N, Page ZA, Dolinski ND, Diaz YJ, Tomlinson W, Clark KD, Hooper JP, Hawker C, Read de Alaniz J. J Am Chem Soc, 2016, 138: 13960–13966
Broman SL, Petersen MÅ, Tortzen CG, Kadziola A, Kilså K, Nielsen MB. J Am Chem Soc, 2010, 132: 9165–9174
Zweig JE, Newhouse TR. J Am Chem Soc, 2017, 139: 10956–10959
Petermayer C, Dube H. J Am Chem Soc, 2018, 140: 13558–13561
Wei P, Zhang JX, Zhao Z, Chen Y, He X, Chen M, Gong J, Sung HHY, Williams ID, Lam JWY, Tang BZ. J Am Chem Soc, 2018, 140: 1966–1975
Tian H, Zhang J. Photochromic Materials. Weinheim: John Wiley & Sons, 2016
Tylkowski B, Jastrzab R, Skrobanska M. Photo-sensitive complexes based on azobenzene. In: Jastrzab R, Tylkowski B, Eds. New-Generation Bioinorganic Complexes. Berlin: De Gruyter, 2016
Li J, Nagamani C, Moore JS. Acc Chem Res, 2015, 48: 2181–2190
Mei X, Wei K, Wen G, Liu Z, Lin Z, Zhou Z, Huang L, Yang E, Ling Q. Dyes Pigments, 2016, 133: 345–353
Ko CC, Kwok WM, Yam VWW, Phillips DL. Chem Eur J, 2006, 12: 5840–5848
Elsner C, Cordes T, Dietrich P, Zastrow M, Herzog TT, Ruck-Braun K, Zinth W. J Phys Chem A, 2009, 113: 1033–1039
Shorunov SV, Krayushkin MM, Stoyanovich FM, Irie M. Russ J Org Chem, 2006, 42: 1490–1497
Lee S, You Y, Ohkubo K, Fukuzumi S, Nam W. Org Lett, 2012, 14: 2238–2241
Meng X, Zhu W, Zhang Q, Feng Y, Tan W, Tian H. J Phys Chem B, 2008, 112: 15636–15645
Peng S, Sun R, Wang W, Chen C. Angew Chem Int Ed, 2017, 56: 6882–6885
Luo X, Qian L, Xiao Y, Tang Y, Zhao Y, Wang X, Gu L, Lei Z, Bao J, Wu J, He T, Hu F, Zheng J, Li H, Zhu W, Shao L, Dong X, Chen D, Qian X, Yang Y. Nat Commun, 2019, 10: 258
Grimm JB, Brown TA, Tkachuk AN, Lavis LD. ACS Cent Sci, 2017, 3: 975–985
Fischer C, Sparr C. Angew Chem Int Ed, 2018, 57: 2436–2440
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE. Chem Rev, 1997, 97: 1515–1566
Fukumoto S, Nakashima T, Kawai T. Angew Chem Int Ed, 2011, 50: 1565–1568
Erko FG, Berthet J, Patra A, Guillot R, Nakatani K, Métivier R, Delbaere S. Eur J Org Chem, 2013, 2013: 7809–7814
Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Science, 2006, 313: 1642–1645
Rust MJ, Bates M, Zhuang X. Nat Methods, 2006, 3: 793–796
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21822805, 21922704, 21877069, 21908065, 22078098), China Postdoctoral Science Foundation (2019M651427, 2020T130197) and the Commission of Science and Technology of Shanghai Municipality (18430711000).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Gu, L., Zhang, L., Luo, X. et al. The mechanodonor-acceptor coupling (MDAC) approach for unidirectional multi-state fluorochromism. Sci. China Chem. 64, 253–262 (2021). https://doi.org/10.1007/s11426-020-9874-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-020-9874-6