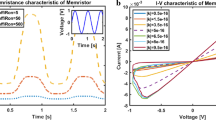
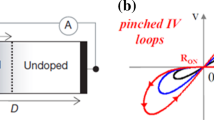
Abstract
Knowing the connectivity patterns in neural circuitry is essential to understand the operating mechanism of the brain, as it allows the analysis of how neural signals are processed and flown through the neural system. With the recent advances in neural recording technologies in terms of channel size and time resolution, a simple and efficient system to perform neural connectivity inference is highly desired, which will enable the process of high dimensional neural activity recording data and reduction of the computational time and cost. In this work, we show that the spike-timing dependent plasticity (STDP) algorithm can be used to reconstruct neural connectivity patterns in a biological neural network, with higher accuracy and efficiency than statistic-based inference methods. The biologically inspired STDP learning rules are natively implemented in a second-order memristor network and are used to estimate the type and the direction of neural connections. When stimulated by the recorded neural spike trains, the memristor device conductance is modulated by the proposed STDP learning rules, which in turn reflects the correlation of the spikes and the possibility of neural connections. By compensating for the different levels of neural activity, highly reliable inference performance can be achieved. The proposed approach offers real-time and local learning, resulting in reduced computational cost/time and strong tolerance to variations of the neural system.
Similar content being viewed by others
References
Insel T R, Landis S C, Collins F S. The NIH brain initiative. Science, 2013, 340: 687–688
Amunts K, Ebell C, Muller J, et al. The human brain project: creating a European research infrastructure to decode the human brain. Neuron, 2016, 92: 574–581
Okano H, Sasaki E, Yamamori T, et al. Brain/MINDS: a Japanese national brain project for marmoset neuroscience. Neuron, 2016, 92: 582–590
Brown E N, Kass R E, Mitra P P. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci, 2004, 7: 456–461
Pillow J W, Shlens J, Paninski L, et al. Spatio-temporal correlations and visual signaling in a complete neuronal population. Nature, 2008, 454: 995–999
de Abril I M, Yoshimoto J, Doya K. Connectivity inference from neural recording data: challenges, mathematical bases and research directions. Neural Netw, 2018, 102: 120–137
Garofalo M, Nieus T, Massobrio P, et al. Evaluation of the performance of information theory-based methods and cross-correlation to estimate the functional connectivity in cortical networks. PLoS ONE, 2009, 4: 6482
Salinas E, Sejnowski T J. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci, 2001, 2: 539–550
Kraskov A, Stögbauer H, Grassberger P. Estimating mutual information. Phys Rev E, 2004, 69: 066138
Schreiber T. Measuring information transfer. Phys Rev Lett, 2000, 85: 461–464
Ito S, Hansen M E, Heiland R, et al. Extending transfer entropy improves identification of effective connectivity in a spiking cortical network model. PLoS ONE, 2011, 6: 27431
Pastore V P, Massobrio P, Godjoski A, et al. Identification of excitatory-inhibitory links and network topology in large-scale neuronal assemblies from multi-electrode recordings. PLoS Comput Biol, 2018, 14: 1006381
Kobayashi R, Kurita S, Kurth A, et al. Reconstructing neuronal circuitry from parallel spike trains. Nat Commun, 2019, 10: 1–13
Kim S, Choi S H, Lu W. Comprehensive physical model of dynamic resistive switching in an oxide memristor. ACS Nano, 2014, 8: 2369–2376
Kim S, Du C, Sheridan P, et al. Experimental demonstration of a second-order memristor and its ability to biorealistically implement synaptic plasticity. Nano Lett, 2015, 15: 2203–2211
Lee S H, Moon J, Jeong Y J, et al. Quantitative, Dynamic TaOx memristor/resistive random access memory model. ACS Appl Electron Mater, 2020, 2: 701–709
Bi G Q, Poo M M. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci, 1998, 18: 10464–10472
Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci, 2008, 31: 25–46
Diehl P U, Cook M. Unsupervised learning of digit recognition using spike-timing-dependent plasticity. Front Comput Neurosc, 2015, 9: 99
Kheradpisheh S R, Ganjtabesh M, Thorpe S J, et al. STDP-based spiking deep convolutional neural networks for object recognition. Neural Netw, 2018, 99: 56–67
Gewaltig M O, Diesmann M. NEST (neural simulation tool). Scholarpedia, 2007, 2: 1430
Song S, Sjöström P J, Reigl M, et al. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol, 2005, 3: 68
Hoffmann J H O, Meyer H S, Schmitt A C, et al. Synaptic conductance estimates of the connection between local inhibitor interneurons and pyramidal neurons in layer 2/3 of a cortical column. Cereb Cortex, 2015, 25: 4415–4429
Matthews B W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim Biophys Acta (BBA) Protein Structure, 1975, 405: 442–451
Zhu X, Wang Q, Lu W D. Memristor networks for real-time neural activity analysis. Nat Commun, 2020, 11: 2439
Acknowledgements
This work was supported in part by National Science Foundation through Award (Grant No. 1915550). The authors would like to thank Dr. S. H. Lee and Dr. M. Zidan for stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moon, J., Wu, Y., Zhu, X. et al. Neural connectivity inference with spike-timing dependent plasticity network. Sci. China Inf. Sci. 64, 160405 (2021). https://doi.org/10.1007/s11432-021-3217-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11432-021-3217-0