Abstract
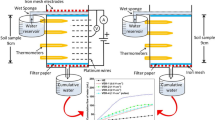
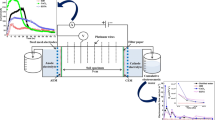
The direction of electroosmotic flow in clay is normal from the anode to the cathode, and the opposite direction is rarely observed. However, electroosmotic flow from the cathode to the anode was observed in kaolin acidified to pH 4 by acetic acid during an electroosmosis experiment. It had an impact on the electrokinetic remediation occurring with lead-contaminated kaolin. The experimental results indicated that reverse electroosmotic flow from the cathode to the anode was caused by a decrease in the absolute value of the soil zeta potential due to the compressed double electric layer and the hydrophilic carboxyl groups in acetate ions. The reverse electroosmotic flow was stronger than normal electroosmotic flow from anode to cathode. The reverse electroosmotic flow had an impact on migration of lead ions in the lead-contaminated kaolin during electrokinetic remediation experiments. The experimental results indicated that the rate for removal of Pb was increased by 10.1% due to the reverse electroosmotic flow. A micro-mechanism model for lead ion migration was built according to the functions of electric field and electroosmotic flows on lead ions, and it explained well the mechanism for the impact of reverse electroosmosis on lead ion removal. The micro-mechanism model indicated that when the direction of the stronger electroosmotic flow is the same as the migration direction of Pb, the rate for removal of Pb is improved, and vice versa.
















Similar content being viewed by others
References
Zhang W, Yang G, Tu X et al (1999) Determination of pH value in forest soil LYT1239–1999. Chinese Academy of Forestry, Beijing
Xia J, Cai D, Xia Z et al (1995) Environmental quality standard for soils GB15618–1995. National Environmental Protection Agency, Beijing
Jin Y, E X, Chen C et al (2006) Standards for drinking water quality GB5749–2006. National Institute of Environment Health, China CDC, Beijing
Acar YB, Alshawabkeh AN (1993) Principles of electrokinetic remediation. Environ Sci Tech 27(13):2638–2647
Alcántara MT, Gómez J, Pazos M et al (2012) Electrokinetic remediation of lead and phenanthrene polluted soils. Geoderma 173–174:128–133. https://doi.org/10.1016/j.geoderma.2011.12.009
Azzam R, Oey W (2001) The utilization of electrokinetics in geotechnical and environmental engineering. Transp Porous Media 42:293–314. https://doi.org/10.1023/A:1006753622691
Bao H (2010) Analytical chemistry. Higher Education Press, Beijing, pp 135–137
Cai Z, Yang H, Cheng R (2008) Hydration number of carboxylic acid ions in water. Acta Chim Sinica 66:831–833
Cameselle C, Pena A (2016) Enhanced electromigration and electro-osmosis for the remediation of an agricultural soil contaminated with multiple heavy metals. Process Saf Environ 104:209–217. https://doi.org/10.1016/j.psep.2016.09.002
Cameselle C, Reddy KR (2012) Development and enhancement of electro-osmotic flow for the removal of contaminants from soils. Electrochim Acta 86:10–22
Wu C, Yuan S, Wan J et al (2008) The electromigration of cadmium in contaminated kaolin by a primary cell. Environ Chem 2:168–171
Min F, Qing Z, Li H et al (2013) Study of electrokinetic properties of kaolinite in coal slime. J China Univ Min Technol 02:127–133. https://doi.org/10.13247/j.cnki.jcumt.2013.02.020
Zeng G, Gao Y (1956) Electrochemical strengthening of soft clay (preliminary test results). J Zhejiang Univ (Eng Sci) 02:15–38.
Gustafsson JP (2012) Visual MINTEQ 3.0 user guide. Dep.of Land & Water Resour.eng
Jalilehvand F, Spngberg D, Lindqvist-Reis P et al (2001) Hydration of the calcium ion. An EXAFS, large-angle X-ray scattering, and molecular dynamics simulation study. J Am Chem Soc 123:431–441. https://doi.org/10.1021/ja001533a
Jo SU, Kim DH, Yang JS et al (2012) Pulse-enhanced electrokinetic restoration of sulfate-containing saline greenhouse soil. Electrochim Acta 86:57–62
Kaya A, Yukselen Y (2011) Zeta potential of clay minerals and quartz contaminated by heavy metals. Revue Canadienne De Géotechnique 42:1280–1289. https://doi.org/10.1139/t05-048
Kimura T, Takase KI, Tanaka S (2007) Concentration of copper and a copper-EDTA complex at the pH junction formed in soil by an electrokinetic remediation process. J Hazard Mater 3:668–672
Lee KY, Kim KW (2010) Heavy metal removal from shooting range soil by hybrid electrokinetics with bacteria and enhancing agents. Environ Sci Technol 44:9482–9487
Lei Y, Kai X (2016) Accurate calculation of buffer capacity. Guangzhou Chem Ind 44:131–132
Lu X, Huang X, Cheng J et al (2007) Study of the matrix effects of simulated soil components(kaoline and montmorillonite) on the electrokinetic remediation for Cu(II) contaminated soils. Sciencepaper Online 2:577–581
Méndez E, Pérez M, Romero O et al (2012) Effects of electrode material on the efficiency of hydrocarbon removal by an electrokinetic remediation process. Electrochim Acta 86:148–156
Mitchell JK, Mitchell J, Mitchell J et al (2005) Fundamentals of Soil Behaviour, pp 282–283
Pamukcu S, Weeks A, Wittle JK (2004) Enhanced reduction of Cr(VI) by direct electric current in a contaminated clay. Environ Sci Technol 38:1236–1241
Pomi R, Masi M, Marini A et al (2015) Electrokinetic remediation of metal-polluted marine sediments: experimental investigation for plant design. Electrochim Acta 181:146–159. https://doi.org/10.1016/j.electacta.2015.04.093
Reddy K, Chinthamreddy S (2004) Enhanced electrokinetic remediation of heavy metals in glacial till soils using different electrolyte solutions. J Environ Eng 130:442–455. https://doi.org/10.1061/(ASCE)0733-9372(2004)130:4(442)
Reddy KR, Chinthamreddy S (2015) Electrokinetic remediation of heavy metal-contaminated soils under reducing environments. Waste Manage 19:269–282
Reginatto AR, Ferrero JC (1975) Collapse potential of soils and soil-water chemistry. Int J Rock Mech Min Sci Geomech Abstr 12:59
Rojo A, Hansen HK, Agramonte M (2011) Electrokinetic remediation with high frequency sinusoidal electric fields. Sep Purif Technol 79:139–143
Song Y, Ammami MT, Benamar A et al (2016) Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environ Sci Pollut Res 23:10577–10586. https://doi.org/10.1007/s11356-015-5966-5
Suzuki T, Niinae M, Koga T et al (2014) EDDS-enhanced electrokinetic remediation of heavy metal-contaminated clay soils under neutral pH conditions. Colloids Surf, A 440:145–150
Vizcaíno RL, Yustres A, León MJ et al (2017) Multiphysics implementation of electrokinetic remediation models for natural soils and porewaters. Electrochim Acta 225:93–104. https://doi.org/10.1016/j.electacta.2016.12.102
Villen GM, Garcia RA, Paz-Garcia JM et al (2015) The use of ethylenediaminetetraacetic acid as enhancing agent for the remediation of a lead polluted soil. Electrochim Acta 181:82–89. https://doi.org/10.1016/j.electacta.2015.03.061
Wang J, Zhang DS, Stabnikova O et al (2005) Evaluation of electrokinetic removal of heavy metals from sewage sludge. J Hazard Mater 124:139–146. https://doi.org/10.1016/j.jhazmat.2005.04.036
Chao W, Xia Y(1991) Electrolyte. Xi’an Jiaotong University Press, pp 52
Gan W, He Y, Zhang X et al (2012) Speciation analysis of heavy metals in soils polluted by electroplating and effect of washing to the removal of the pollutants. J Ecol Rural Environ 28:82–87
Yang C, Li J, Cang L (2008) A review: electrokinetic remediation technology and its applications for heavy metals removal from Sewage Sludge. Water Purif Technol 27:1–4
Yang GCC (2019) Integrated electrokinetic processes for the remediation of phthalate esters in river sediments: A mini-review. Sci Total Environ 659:963–972. https://doi.org/10.1016/j.scitotenv.2018.12.334
Yeung AT, Gu YY (2011) A review on techniques to enhance electrochemical remediation of contaminated soils. J Hazard Mater 195:11–29
Yuan L, Li H, Xu X et al (2016) Electrokinetic remediation of heavy metals contaminated kaolin by a CNT-covered polyethylene terephthalate yarn cathode. Electrochim Acta 213:140–147. https://doi.org/10.1016/j.electacta.2016.07.081
Yukselen Y, Kaya A (2003) Zeta potential of kaolinite in the presence of alkali, alkaline earth and hydrolyzable metal ions. Water Air Soil Pollut 145:155–168. https://doi.org/10.1023/A:1023684213383
Zhou J, Lu X, Wang Y et al (2000) Molecular dynamics simulationof ionic hydration. J Chem Ind Eng 21:762–765
Zhuang YF, Zou W, Wang Z et al (2012) Electrically conductive PVD No. ZL201210197981.4.
Acknowledgements
This work was supported by research grants from National Key Research and Development Program of China (Grant No. 2021YFC2901701) and the National Natural Science Foundation of China (NSFC Grant Nos. 41472039 and 51109168).
Author information
Authors and Affiliations
Contributions
Yan-feng Zhuang led the projects and designed the experiments. Zhitao Liu participated in the experiment design and carried out the experiments. Yani Liu participated in the experiments, carried out the data analysis and drafted the manuscript. Fang Xiao participated in preparation and revision of the manuscript; Yan-feng Zhuang significantly helped with revision of the manuscript and gave final approval for the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Zhuang, Yf., Xiao, F. et al. Mechanism for reverse electroosmotic flow and its impact on electrokinetic remediation of lead-contaminated kaolin. Acta Geotech. 18, 1515–1528 (2023). https://doi.org/10.1007/s11440-022-01640-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11440-022-01640-3