Abstract
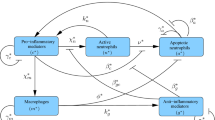
There is growing interest in inflammation due to its involvement in many diverse medical conditions, including Alzheimer’s disease, cancer, arthritis and asthma. The traditional view that resolution of inflammation is a passive process is now being superceded by an alternative hypothesis whereby its resolution is an active, anti-inflammatory process that can be manipulated therapeutically. This shift in mindset has stimulated a resurgence of interest in the biological mechanisms by which inflammation resolves. The anti-inflammatory processes central to the resolution of inflammation revolve around macrophages and are closely related to pro-inflammatory processes mediated by neutrophils and their ability to damage healthy tissue. We develop a spatially averaged model of inflammation centring on its resolution, accounting for populations of neutrophils and macrophages and incorporating both pro- and anti-inflammatory processes. Our ordinary differential equation model exhibits two outcomes that we relate to healthy and unhealthy states. We use bifurcation analysis to investigate how variation in the system parameters affects its outcome. We find that therapeutic manipulation of the rate of macrophage phagocytosis can aid in resolving inflammation but success is critically dependent on the rate of neutrophil apoptosis. Indeed our model predicts that an effective treatment protocol would take a dual approach, targeting macrophage phagocytosis alongside neutrophil apoptosis.















Similar content being viewed by others
References
Akgul C, Moulding DA, Edwards SW (2001) Molecular control of neutrophil apoptosis. FEBS Lett 487:318–322
Alt W, Lauffenburger D (1985) Transient behaviour of a chemotaxis system modelling certain types of tissue inflammation. J Math Biol 24:691–722
Butterfield A, Best T, Merrick M (2006) The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train 41:457–465
Cao C, Lawrence DA, Strickland DK, Zhang L (2005) A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 106:32343241
Eming S, Kreig T, Davidson J (2007) Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127:514–525
Gilroy DW, Lawrence T, Perretti M, Rossi AG (2004) Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov 3(5):401–16
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Immunology 5:953–964
Haslett C (1999) Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med 160:5–11
Heasman SJ, Giles KM, Ward C, Rossi AG, Haslett C, Dransfield I (2003) Mechanisms of steroid action and resistance in inflammation. Glucocorticoid-mediated regulation of granulocyte apoptosis and macrophage phagocytosis of apoptotic cells: implication for the resolution of inflammation. J Endocrinol 178:29–36
Henson PM (2005) Dampening inflammation. Nat Immunol 6:1179–1181
Kobayashi SD, DeLeo FR (2009) Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscipl Rev 1:309–333. doi:10.1002/wsbm.32
Kobayashi SD, Voyich J, DeLeo FR (2003) Regulation of the neutrophil-mediated inflammatory response to infection. Microbes Infect 5:1337–1344
Kumar R, Clermont G, Vodovotz Y, Chow C (2004) The Dynamics of Acute Inflammation. J Theor Biol 230:145–155
Lauffenburger D, Keller K (1979) Effects of leukocyte random motility and chemotaxis in tissue inflammatory response. J Theor Biol 81:475–503
Lauffenburger D, Kennedy C (1983) Localized bacterial infection in a distribute model for tissue inflammation. J Math Biol 16:141–163
Lauffenburger D, Kennedy C (1981) Analysis of a lumped model for tissue inflammation dynamics. J Math Biosci 53:189–221
Lawrence T, Gilroy DW (2007) Chronic inflammation: a failure of resolution? Int J Exp Path 88:85–94
Lawrence T, Willoughby DA, Gilroy DW (2007) Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Annu Rev Immunol 25:101–37
Lee A, Whyte MK, Haslett C (1993) Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol 54:283–288
Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR (2012) Neutrophils in tuberculosis: friend or foe? Trends Immunol 33:1425
Luster AD, Alon R, von Andrian UH (2005) Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol 6:1182–1190
Maderna P, Godson C (2003) Phagocytosis of apoptectic cells and the resolution of inflammation. Biochim Biophys Acta 1639:141–151
Mare AFM, Komba F, Dyck C, Labecki M, Finegood DT, Edelstein-Keshet L (2005) Quantifying macrophage defects in type 1 diabetes. J Theor Biol 233:533–551
Mcsai A (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. JEM 210(7):1283–1299
Murray JD (2003) Mathematical biology II: spatial models and biomedical applications. Springer, Berlin
Nathan C (2002) Points of control in inflammation. Nature 420:846–852
Parihar A, Eubank TD, Doseff AI (2010) Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun 2:204–215
Porcheray F, Viaud S, Rimmaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142:481–489
Reid C, Rushe M, Jarpe M, van Vlijmen H, Dolinski B, Qian F, Cachero T, Cuervo H, Yanachkova M, Nwankwo C, Wang X, Etienne N, Garber E, Bailly V, de Fougerolles A, Boriack-Sjodin P (2006) Structure activity relationships of monocyte chemoattractant proteins in complex with a blocking antibody. Protein Eng Des Sel 19:317–324
Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, Ermentrout G (2006) A reduced mathematical model of the acute inflammatory response I. Derivation of model and analysis of anti-inflammation. J Theor Biol 242:220–236
Rossi AG, Hallett JM, Sawatzky DA, Teixeira MM, Haslett C (2007) Modulation of granulocyte apoptosis can influence the resolution of inflammation. Biochem Soc Trans 35:288–291
Rossi A, Sawatzky D (2008) The resolution of inflammation (Progress in inflammation research). Birkhauser Verlag AG, Basel
Scott A, Kham KM, Roberts CR, Cook JL, Duronio V (2004) What do we mean by the term ”inflammation”? A contemporary basic science update for sports medicine. Br J Sports Med 38:372–380
Serhan CN (2007) Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 25:101–137
Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J 21:325–330
Serhan CN, Savill J (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197
Smith AM, McCullers JA, Adler FR (2011) Mathematical model of a three-stage innate immune response to a pneumococcal lung infection. J Theor Biol 276:106–116
Stout RD, Jiang C, Matta B, Tietzel I, Watkins S, Suttles J (2005) Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175:342–349
Su B, Zhou W, Dorman KS, Jones DE (2009) Mathematical modelling of immune response in tissues. Comput Math Methods Med 10:9–38
Szpaderska AM, DiPietro LA (2005) Inflammation is surgical wound healing: friend or foe? Surgery 137:571–573
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288:345–353
Vodovotz Y (2006) Deciphering the complexity of acute inflammation using mathematical models. Immunol Res 36:237–245
Ward C, Dransfield I, Chilvers ER, Haslett C, Rossi AG (1999) Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol Sci 20:503–509
Waugh HV, Sherratt JA (2007) Modeling the effects of treating diabetic wounds with engineered skin substitutes. Wound Rep Reg 15:556–565
Wigginton JE, Kirschner D (2001) A model to predict cell-mediated immune regulatory mechanisms during human infection with mycobacterium tuberculosis. J Immunol 166:1951–1967
Acknowledgments
JLD gratefully acknowledges support from the Engineering and Physical Sciences Research Council, the Health and Safety Laboratory and the industrial mathematics KTN for this work in the form of a CASE studentship. JRK acknowledges the funding of the Royal Society and Wolfson Foundation. The work of HMB was supported in part by Award No. KUK-013-04, made by King Abdullah University of Science and Technology (KAUST).
Author information
Authors and Affiliations
Corresponding author
Parameter Values
Parameter Values
In this appendix, we discuss the values of the dimensional parameters and nondimensional parameter groups. Obtaining precise values for parameters for such biological processes is difficult for several reasons. Some biological mechanisms in inflammation remain unclear (Haslett 1999); measurements of acute inflammatory markers in vivo are difficult to obtain because acute inflammation is typically short-lived and patients often do not present until the condition has progressed beyond the acute stage. Further the parameter values can vary between tissue types; for example tendon, muscles and lung have different structures and patterns of vascularisation. Where possible, estimates are taken from the cited literature, and extra weight attached to data from humans or human cells and soft-tissue-specific data relative to other sources. If no data are available then order of magnitude estimates that give biologically realistic results are employed.
Decay and death rates are readily available in literature. Extracellular mediator decay rates are reported to lie in the range 0.7–20 per day (Smith et al. 2011; Waugh and Sherratt 2007; Su et al. 2009). Accordingly we fix \(\gamma _c=3\) day\(^{-1}\) for pro-inflammatory mediator decay. We expect our two generic mediators to have a similar half-life (\(\gamma _c\sim \gamma _g\)) and neutrophils are reported to undergo secondary necrosis (\(\tilde{\gamma _a}\)) within a few hours of their death by apoptosis (Haslett 1999). Accordingly we set
Under normal conditions, neutrophils are known to have a short life dying within days (Akgul et al. 2001) while macrophages are resistant to apoptotic stimuli therefore living longer (Parihar et al. 2010), often for several weeks, before leaving the inflammatory site via the lymphatics (Serhan and Savill 2005). Accordingly we set
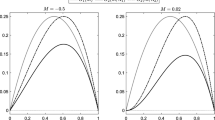
We can estimate from the literature the rate at which macrophages engulf cells (Mare et al. 2005; Wigginton and Kirschner 2001) (allowing us to set \(\phi =1\times 10^{-3}\) mm\(^3\) cell\(^{-1}\) day\(^{-1}\)). We have estimates of the rate of production of TGF\(_\beta \) by macrophages of 0.07 pg cell\(^{-1}\) day\(^{-1}\) (Waugh and Sherratt 2007). The production of a generic anti-inflammatory mediator (such as TGF\(_\beta \)) is captured in our model by the product of parameters \(k_g\) (the rate that macrophages produce anti-inflammatory mediator) and \(\phi \) (the rate at which macrophages engulf cells) allowing us to fix \(k_g\) so that \(k_g\,\phi =0.07\) pg cell\(^{-1}\) day\(^{-1}\). In the context of this model there is no available data for the rates of influx of neutrophils and macrophages (though we know that neutrophils arrive much quicker than macrophages (Butterfield et al. 2006)) or for the production of generic pro-inflammatory mediators (\(k_a\), \(k_n\)) or saturation constants (\(\beta _c\), \(\beta _{gc}\), \(\beta _g\)). The remaining nondimensional parameters are based on groupings of known parameters and those for which no data are available (for example \(\tilde{\phi }=\phi \chi _m k_a / \gamma _c^2\)). Since such groupings are difficult to estimate we use values based on biologically realistic results so that
Rights and permissions
About this article
Cite this article
Dunster, J.L., Byrne, H.M. & King, J.R. The Resolution of Inflammation: A Mathematical Model of Neutrophil and Macrophage Interactions. Bull Math Biol 76, 1953–1980 (2014). https://doi.org/10.1007/s11538-014-9987-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-9987-x