Abstract
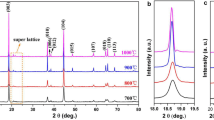
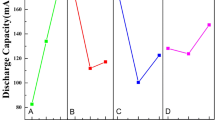
A crystalline structure of LiCoO2 sample was synthesized at different stirring times via sol-gel method. This was followed by the electrochemical characterization of LiCoO2 in 5 M LiNO3 aqueous electrolyte. The hexagonal LiCoO2 was stirred for 30 h produced the highest peak intensity and smallest particle size. A morphological analysis showed the particle size distribution within the range of 0.32–0.47 μm. At lower scan rates of cyclic voltammetry, three pairs of redox peaks at ESCE = 0.81/0.65, 0.89/0.83 and 1.01/0.95 V were observed. The peak separation was proportionally consistent with Li+ diffusion coefficients of 7.42 × 10−8 cm2 s−1 (anodic) and 3.59 × 10−8 cm2 s−1 (cathodic). For specific capacity, the LiCoO2 demonstrated a higher initial specific capacity (115.49 mA h g−1). A small difference (1.92 Ω) in the charge transfer resistance before and after a charge discharge analysis indicated that the Li+ ions had been well-diffused during the intercalation/de-intercalation process.







Similar content being viewed by others
References
Liu XH, Saito T, Doi T, Okada S, Yamaki JI (2009) Electrochemical properties of rechargeable aqueous lithium ion batteries with an olivine-type cathode and a Nasicon-type anode. J Power Sources 189:706–710
Ruffo R, La Mantia F, Wessells C, Huggins RA, Cui Y (2011) Electrochemical characterization of LiCoO2 as rechargeable electrode in aqueous LiNO3 electrolyte. Solid State Ionics 192:289–292
Alias N, Mohamad AA (2015) Advances of aqueous rechargeable lithium-ion battery: a review. J Power Sources 274:237–251
Wang GJ, Qu QT, Wang B, Shi Y, Tian S, Wu YP, Holze R (2009) Electrochemical behavior of LiCoO2 in a saturated aqueous Li2SO4 solution. Electrochim Acta 54:1199–1203
Guan T, Zuo P, Sun S, Du C, Zhang L, Cui Y, Yang L, Gao Y, Yin G, Wang F (2014) Degradation mechanism of LiCoO2/mesocarbon microbeads battery based on accelerated aging tests. J Power Sources 268:816–823
Asgari S, Soltanmohammad S (2010) Characterization of LiCoO2 nanopowders produced by sol-gel processing. J Nanomater 8:1–8. doi:10.1155/2010/104012
Predoana L, Jitianu A, Preda S, Malic B, Zaharescu M (2015) Thermal behavior of Li–Co-citric acid water-based gels as precursors for LiCoO2 powders. J Therm Anal Calorim 119:145–153
Zhu C, Yang C, Yang W-D, Hsieh C-Y, Ysai H-M, Chen Y-S (2010) High performances of ultrafine and layered LiCoO2 powders for lithium batteries by a novel sol–gel process. J Alloys Compd 496:703–709
Heli H, Yadegari H, Jabbari A (2012) A study of the lithium intercalation into nanoparticles of LiCoO2 from an aqueous solution. J Appl Electrochem 42:279–289
Ou Y, Wen J, Xu H, Xie S, Li J (2013) Ultrafine LiCoO2 powders derived from electrospun nanofibers for Li-ion batteries. J Phys Chem Solids 74:322–327
Alias N, Mohamad AA (2014) Synthesis and electrochemical behavior of LiFePO4/C with an air–electrode in an aqueous lithium ion battery. Ceram Int 40(8, Part B):13089–13096
Tang W, Liu LL, Tian S, Li L, Yue YB, Wu YP, Guan SY, Zhu K (2010) Nano-LiCoO2 as cathode material of large capacity and high rate capability for aqueous rechargeable lithium batteries. Electrochem Commun 12(11):1524–1526
Qu Q, Fu L, Zhan X, Samuelis D, Maier J, Li L, Tian S, Li Z, Wu Y (2011) Porous LiMn2O4 as cathode material with high power and excellent cycling for aqueous rechargeable lithium batteries. Energy Environ Sci 4:3985–3990
Predoană L, Barău A, Zaharescu M, Vassilchina H, Velinova N, Banov B, Momchilov A (2007) Electrochemical properties of the LiCoO2 powder obtained by sol–gel method. J Eur Ceram Soc 27:1137–1142
Zeng XL, Huang YY, Luo FL, He YB, Tong DG (2010) Synthesis of LiCoO2 by l-apple acid assisted sol–gel method and its electrochemical behavior in aqueous lithium-ion battery. J Sol-Gel Sci Technol 54:139–146
Mei T, Tang K, Zhu Y, Qian Y (2011) Preparation of LiCoO2 concaved cuboctahedra and their electrochemical behavior in lithium-ion battery. Dalton Trans 40:7645–7650
Yang W-D, Hsieh C-Y, Chuang H-J, Chen Y-S (2010) Preparation and characterization of nanometric-sized LiCoO2 cathode materials for lithium batteries by a novel sol–gel method. Ceram Int 36:135–140
Lala SM, Montoro LA, Lemos V, Abbate M, Rosolen JM (2005) The negative and positive structural effects of Ga doping in the electrochemical performance of LiCoO2. Electrochim Acta 51:7–13
Mirjalili F, Mohamad H, Abdullah LC (2010) Size-controlled synthesis of nano a-alumina particles through the sol–gel method. Ceram Int 36:1253–1257
Zhu X, Shang K, Jiang X, Ai X, Yang H, Cao Y (2014) Enhanced electrochemical performance of mg-doped LiCoO2 synthesized by a polymer-pyrolysis method. Ceram Int 40:11245–11249
Reddy M, Jie TW, Jafta CJ, Ozoemena KI, Mathe MK, Nair AS, Peng SS, Idris MS, Balakrishna G, Ezema FI (2014) Studies on bare and Mg-doped LiCoO2 as a cathode material for lithium ion batteries. Electrochim Acta 128:192–197
Myung S-T, Amine K, Sun Y-K (2015) Nanostructured cathode materials for rechargeable lithium batteries. J Power Sources 283:219–236
Hudak NS, Davis LE, Nagasubramanian G (2015) Cycling-induced changes in the entropy profiles of lithium cobalt oxide electrodes. J Electrochem Soc 162(3):A315–A321
Yang S, Cui G, Pang S, Cao Q, Kolb U, Feng X, Maier J, Müllen K (2010) Fabrication of cobalt and cobalt oxide/graphene composites: towards high-performance anode materials for lithium ion batteries. ChemSusChem 3:236–239
Yadegari H, Jabbari A, Heli H (2012) An aqueous rechargeable lithium-ion battery based on LiCoO2 nanoparticles cathode and LiV3O8 nanosheets anode. J Solid State Electrochem 16:227–234
Tang W, Zhu Y, Hou Y, Liu L, Wu Y, Loh KP, Zhang H, Zhu K (2013) Aqueous rechargeable lithium batteries as an energy storage system of superfast charging. Energy Environ Sci 6:2093–2104
Wang X, Qu Q, Hou Y, Wang F, Wu Y (2013) An aqueous rechargeable lithium battery of high energy density based on coated Li metal and LiCoO2. Chem Commun 49:6179–6181
Wang GJ, Zhang HP, Fu LJ, Wang B, Wu YP (2007) Aqueous rechargeable lithium battery (ARLB) based on LiV3O8 and LiMn2O4 with good cycling performance. Electrochem Commun 9:1873–1876
Luo J-Y, Cui W-J, He P, Xia Y-Y (2010) Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat Chem 2:760–765
Fan X, Luo J, Shao C, Zhou X-s, Niu Z (2015) Electrochemical performance of microdisc-shaped carbon-coated lithium iron phosphate with preferentially exposed (0 1 0) planes in lithium sulfate aqueous solution. Electrochim Acta 158:342–347
Ruffo R, Wessells C, Huggins RA, Cui Y (2009) Electrochemical behavior of LiCoO2 as aqueous lithium-ion battery electrodes. Electrochem Commun 11:247–249
Wang GJ, Zhao NH, Yang LC, Wu YP, Wu HQ, Holze R (2007) Characteristics of an aqueous rechargeable lithium battery (ARLB). Electrochim Acta 52(15):4911–4915
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195:939–954
Needham S, Wang G, Liu H, Drozd V, Liu R (2007) Synthesis and electrochemical performance of doped LiCoO2 materials. J Power Sources 174:828–831
Manjunatha H, Venkatesha TV, Suresh GS (2012) Electrochemical studies of LiMnPO4 as aqueous rechargeable lithium–ion battery electrode. J Solid State Electrochem 16:1941–1952
Wu B, Yufit V, Merla Y, Martinez-Botas RF, Brandon NP, Offer GJ (2015) Differential thermal voltammetry for tracking of degradation in lithium-ion batteries. J Power Sources 273:495–501
Acknowledgements
N.A.A. Aziz would like to thank the Ministry of Higher Education and the Polytechnic for the study leave. The authors would also like to thank MOSTI for their financial support for this project via the Science Fund Grant (03-01-05-SF0621).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 3925 kb)
Rights and permissions
About this article
Cite this article
Abdul Aziz, N.A., Abdullah, T.K. & Mohamad, A.A. Synthesis of LiCoO2 via sol-gel method for aqueous rechargeable lithium batteries. Ionics 24, 403–412 (2018). https://doi.org/10.1007/s11581-017-2225-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2225-4