Abstract
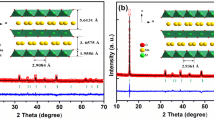
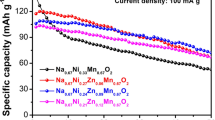
The P2-type Na0.67Mn0.6Fe0.4O2 (NaMnFe), Na0.67Mn0.6Fe0.3Zn0.1O2 (NaMnFeZn), and Na0.67Mn0.6Fe0.2Zn0.1Ni0.1O2 (NaMnFeZnNi) are prepared using an acetate decomposition reaction and developed as promising cathode materials for high-capacity sodium-ion batteries. The XRD patterns show that Zn2+ and Ni2+ ions are successfully incorporated into the lattice of the Na-Mn-Fe-O system, and the P2-type structure remains unchanged after substitution. The charging/discharging tests exhibit that the Na0.67Mn0.6Fe0.4O2, Na0.67Mn0.6Fe0.3Zn0.1O2, and Na0.67Mn0.6Fe0.2Zn0.1Ni0.1O2 electrodes have the capacities of 200.4, 182.0, and 202.2 mAhg−1, respectively. The Na0.67Mn0.6Fe0.4O2 electrode has a higher initial capacity but faster capacity decay. When partially substituting Zn and Ni for Fe, the Na0.67Mn0.6Fe0.3Zn0.1O2 and Na0.67Mn0.6Fe0.2Zn0.1Ni0.1O2 electrodes exhibit lower reversible capacity but improved cycling stability (88.3 and 93.4% capacity retention over 100 cycles). The greatly improved electrochemical performance of the Na0.67Mn0.6Fe0.2Zn0.1Ni0.1O2 electrode apparently belongs to the contribution of the Zn2+ and Ni2+ substitution, which facilitates to alleviate the Jahn-Teller distortion of Mn and suppresses the polarization.








Similar content being viewed by others
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414(6861):359–367. https://doi.org/10.1038/35104644
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334(6058):928–935. https://doi.org/10.1126/science.1212741
Chu S, Majumdar A (2012) Opportunities and challenges for a sustainable energy future. Nature 488(7411):294–303. https://doi.org/10.1038/nature11475
Palomares V, Serras P, Villaluenga I, Hueso KB, González JC, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5(3):5884–5901. https://doi.org/10.1039/c2ee02781j
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-ion batteries. Adv Funct Mater 23(8):947–958. https://doi.org/10.1002/adfm.201200691
Berthelot R, Carlier D, Delmas C (2011) Electrochemical investigation of the P2-NaxCoO2 phase diagram. Nat Mater 10(1):74–80. https://doi.org/10.1038/nmat2920
Yabuuchi N, Yano M, Yoshida H, Kuze S, Komaba S (2013) Synthesis and electrode performance of O3-type NaFeO2-NaNi1/2Mn1/2O2 solid solution for rechargeable sodium batteries. J Electrochem Soc 160(5):A3131–A3137. https://doi.org/10.1149/2.018305jes
Yabuuchi N, Kajiyama M, Iwatate J, Nishikawa H, Hitomi S, Okuyama R, Usui R, Yamada Y, Komaba S (2012) P2-type Nax [Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat Mater 11(6):512–517. https://doi.org/10.1038/nmat3309
Singh G, Acebedo B, M. Cabanas C, Shanmukaraj D, Armand M, Rojo T (2013) An approach to overcome first cycle irreversible capacity in P2-Na2/3[Fe1/2Mn1/2]O2. Electrochem Commun 37:61–63. https://doi.org/10.1016/j.elecom.2013.10.008
Billaud J, Singh G, Armstrong AR, Gonzalo E, Roddatis V, Armand M, Rojo T, Bruce PG (2014) Na0.67Mn1-xMgxO2 (0≤x≤0.2): a high capacity cathode for sodium-ion batteries. Energy Environ Sci 7(4):1387–1391. https://doi.org/10.1039/C4EE00465E
Yabuuchi N, Hara R, Kubota K, Paulsen J, Kumakurad S, Komaba S (2014) A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity. J Mater Chem A 2(40):16851–16855. https://doi.org/10.1039/C4TA04351K
Yabuuchi N, Hara R, Kajiyama M, Kubota K, Ishigaki T, Hoshikawa A, Komaba S (2014) New O2/P2-type Li-excess layered manganese oxides as promising multi-functional electrode materials for rechargeable Li/Na batteries. Adv Energy Mater 4:13072–13072
Yuan D, Hu X, Qian J, Pei F, Wu F, Mao R, Ai X, Yang H, Cao Y (2014) P2-type Na0.67Mn0.65Fe0.2Ni0.15O2 cathode material with high-capacity for sodium-ion battery. Electrochim Acta 116:300–305. https://doi.org/10.1016/j.electacta.2013.10.211
Li Y, Yang Z, Xu S, Mu L, Gu L, Hu Y, Li H, Chen L (2015) Air-stable copper-based P2-Na7/9Cu2/9Fe1/9Mn2/3O2 as a new positive electrode material for sodium-ion batteries. Adv Sci 2(6):1500031. https://doi.org/10.1002/advs.201500031
Buchhol D, Moretti A, Kloepsch R, Nowak S, Siozios V, Winter M, Passerini S (2013) Toward Na-ion batteries synthesis and characterization of a novel high capacity Na ion intercalation material. Chem Mater 44:142–148
Yu X, Xu X, Lee DH, Clement RJ, Leskes M, Pell AJ, Pintacuda G, Yang XQ, Grey CP, Meng YS (2014) Identifying the critical role of Li substitution in P2-Nax(LiyNixMn1-y-z)O2(0<x, y, z<1) intercalation cathode materials for high energy Na-ion batteries. Chem Mater 26:1260–1269
Kubota K, Yoshida H, Yabuuchi N, Ikeuchi I, Garsuch A, Schulz-Dobrick M, Komaba S (2014) P2-type Na2/3Ni1/3Mn2/3−xTixO2 as a new positive electrode for higher energy Na-ion batteries. Chem Commun 50:3677–3680
Wu X, Guo J, Wang D, Zhong G, McDonald MJ, Yang Y (2015) P2-type Na0.66Ni0.33-xZnxMn0.67O2 as new high-voltage cathode materials for sodium-ion batteries. J Power Sources 281:18–26. https://doi.org/10.1016/j.jpowsour.2014.12.083
Buchholz D, Vaalma C, Chagas LG, Passerini S (2015) Mg-doping for improved long-term cyclability of layered Na-ion cathode materials—the example of P2-type NaxMg0.11Mn0.89O2. J Power Sources 282:581–585. https://doi.org/10.1016/j.jpowsour.2015.02.069
Gonzalo E, Han MH, López del Amo JM, Acebedo B, Casas-Cabanas M, Rojo T (2014) Synthesis and characterization of pure P2-and O3-Na2/3Fe2/3Mn1/3O2 as cathode materials for Na ion batteries. J Mater Chem A 2(43):18523–18530. https://doi.org/10.1039/C4TA03991B
Doubaji S, Valvo M, Saadoune I, Dahbi M, Edström K (2014) Synthesis and characterization of a new layered cathode material for sodium ion batteries. J Power Sources 266:275–281. https://doi.org/10.1016/j.jpowsour.2014.05.042
Zhou Y, Wu X, Wu W, Wang K (2014) Synthesis and electrochemical performance of Na0.7Fe0.7Mn0.3O2 as a cathode material for Na-ion battery. Ceram Int 40:13679–13682
Jian Z, Yu H, Zhou H (2013) Designing high-capacity cathode materials for sodium-ion batteries. Electrochem Commun 34:215–218. https://doi.org/10.1016/j.elecom.2013.06.017
Aoki A (1976) X-ray photoelectron spectroscopic studies on ZnS: MnF2 phosphors. Jpn J Appl Phys 15(2):305–311. https://doi.org/10.1143/JJAP.15.305
Allen GC, Curtis MT, Hooper AJ, Tucker PM (1974) X-ray photoelectron spectroscopy of iron-oxygen systems. J Chem Soc Dalton Trans 14:1525–1530. https://doi.org/10.1039/DT9740001525
Pan L, Xia Y, Qiu B, Zhao H, Guo H, Jia K, Gu Q, Liu Z (2016) Structure and electrochemistry of B doped Li (Li0.2Ni0.13Co0.13Mn0.54)1-xBxO2 as cathode materials for lithium-ion batteries. J Power Sources 327:273–280. https://doi.org/10.1016/j.jpowsour.2016.07.064
Hu G, Zhang M, Liang L, Peng Z, Du K, Cao Y (2016) Mg-Al-B co-substitution LiNi0.5Co0.2Mn0.3O2 cathode materials with improved cycling performance for lithium-ion battery under high cutoff voltage. Electrochim Acta 190:264–275. https://doi.org/10.1016/j.electacta.2016.01.039
Mcintyre NS, Cook MG (1975) X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal Chem 47(13):2208–2213. https://doi.org/10.1021/ac60363a034
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Zong, J. & Liu, Xj. P2-type Na0.67Mn0.6Fe0.4-x-yZnxNiyO2 cathode material with high-capacity for sodium-ion battery. Ionics 24, 1939–1946 (2018). https://doi.org/10.1007/s11581-018-2442-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2442-5