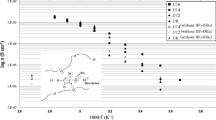
Abstract
Two new plasticizers, nitrile groups terminated oligoether (NOE) and lithium sulfonamide groups containing oligoether (LSA), have been synthesized to construct superior poly(ethylene oxide) (PEO)-based solid polymer electrolytes (SPEs). The chemical structures of these plasticizers were confirmed by FTIR, 1H-NMR, and elemental analysis. The electrochemical and thermal properties of the resulting SPEs have been thoroughly evaluated. The SPEs containing varied weight ratios of these plasticizers along with different Li/O mole ratios of lithium bis(trifluoromethylsulfonyl)imide (LiTFSI) have been investigated to optimize the electrolyte systems. Both plasticizers displayed good ionic conductivity at elevated temperatures. The SPE containing 40 wt.% of NOE in 12:1 PEO-LiTFSI complex showed ionic conductivity of 1.11 × 10−4 S cm−1 at 312 K, while the SPE containing 10 wt.% of LSA in 12:1 PEO-LiTFSI complex showed 5.08 × 10−5 S cm−1 and 1.88 × 10−4 S cm−1 at room temperature and 312 K, respectively. Most notably, the SPE containing 10 wt.% LSA in 14:1 PEO-LiTFSI complex displayed ionic conductivity of 1.01 × 10−3 S cm−1 at 343 K. Moreover, these SPEs exhibited good electrochemical stability (~ 4.2 V vs. Li+/Li) and displayed no noticeable thermal degradations below 350 °C.









Similar content being viewed by others
References
Nair JR, Porcarelli L, Bella F, Gerbaldi C (2015) Newly elaborated multipurpose polymer electrolyte encompassing RTILs for smart energy-efficient devices. ACS Appl Mater Interfaces 7:12961–12971. https://doi.org/10.1021/acsami.5b02729
Jo G, Jeon H, Park MJ (2015) Synthesis of polymer electrolytes based on poly(ethylene oxide) and an anion-stabilizing hard polymer for enhancing conductivity and cation transport. ACS Macro Lett 4:225–230. https://doi.org/10.1021/mz500717j
Rolland J, Brassinne J, Bourgeois JP et al (2014) Chemically anchored liquid-PEO based block copolymer electrolytes for solid-state lithium-ion batteries. J Mater Chem A 2:11839–11846. https://doi.org/10.1039/c4ta02327g
Gerbaldi C, Nair JR, Kulandainathan MA et al (2014) Innovative high performing metal organic framework (MOF)-laden nanocomposite polymer electrolytes for all-solid-state lithium batteries. J Mater Chem A 2:9948–9954. https://doi.org/10.1039/C4TA01856G
Wetjen M, Kim GT, Joost M et al (2014) Thermal and electrochemical properties of PEO-LiTFSI-Pyr 14TFSI-based composite cathodes, incorporating 4 V-class cathode active materials. J Power Sources 246:846–857. https://doi.org/10.1016/j.jpowsour.2013.08.037
Zhang J, Zhao J, Yue L et al (2015) Safety-reinforced poly(propylene carbonate)-based all-solid-state polymer electrolyte for ambient-temperature solid polymer lithium batteries. Adv Energy Mater 5:1–10. https://doi.org/10.1002/aenm.201501082
Colò F, Bella F, Nair JR et al (2015) Cellulose-based novel hybrid polymer electrolytes for green and efficient Na-ion batteries. Electrochim Acta 174:185–190. https://doi.org/10.1016/j.electacta.2015.05.178
Wei Z, Chen S, Wang J et al (2018) A large-size, bipolar-stacked and high-safety solid-state lithium battery with integrated electrolyte and cathode. J Power Sources 394:57–66. https://doi.org/10.1016/j.jpowsour.2018.05.044
Tominaga Y, Yamazaki K (2014) Fast Li-ion conduction in poly(ethylene carbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem Commun 50:4448–4450. https://doi.org/10.1039/c3cc49588d
Huang K-C, Li H-H, Fan H-H et al (2017) An in situ-fabricated composite polymer electrolyte containing large-anion lithium salt for all-solid-state LiFePO4/Li batteries. ChemElectroChem 4:2293–2299. https://doi.org/10.1002/celc.201700322
Li YH, Wu XL, Kim JH et al (2013) A novel polymer electrolyte with improved high-temperature tolerance up to 170 °C for high-temperature lithium-ion batteries. J Power Sources 244:234–239. https://doi.org/10.1016/j.jpowsour.2013.01.148
Xue Z, He D, Xie X (2015) Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A 3:19218–19253. https://doi.org/10.1039/c5ta03471j
Golodnitsky D, Livshits E, Ulus A, Peled E (2002) Highly conductive, oriented polymer electrolytes for lithium batteries. Polym Adv Technol 13:683–689. https://doi.org/10.1002/pat.266
Gray FM, Connor JA (1997) Polymer Electrolytes. Royal Society of Chemistry, London
Gadjourova Z, Andreev YG, Tunstall DP, Bruce PG (2001) Ionic conductivity in crystalline polymer electrolytes. Nature 412:520–523. https://doi.org/10.1038/35087538
He W, Cui Z, Liu X et al (2017) Carbonate-linked poly(ethylene oxide) polymer electrolytes towards high performance solid state lithium batteries. Electrochim Acta 225:151–159. https://doi.org/10.1016/j.electacta.2016.12.113
Zhang J, Wen H, Yue L et al (2017) In situ formation of polysulfonamide supported poly(ethylene glycol) divinyl ether based polymer electrolyte toward monolithic sodium ion batteries. Small 13:1–10. https://doi.org/10.1002/smll.201601530
Cheng S, Smith DM, Li CY (2014) How does nanoscale crystalline structure affect ion transport in solid polymer electrolytes? Macromolecules 47:3978–3986. https://doi.org/10.1021/ma500734q
Zhang J, Ma C, Liu J et al (2016) Solid polymer electrolyte membranes based on organic/inorganic nanocomposites with star-shaped structure for high performance lithium ion battery. J Membr Sci 509:138–148. https://doi.org/10.1016/j.memsci.2016.02.049
Chai J, Liu Z, Ma J et al (2017) In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv Sci 4:1–9. https://doi.org/10.1002/advs.201600377
Olgun U, Gülfen M (2014) Synthesis of fluorescence poly(phenylenethiazolo[5,4-d]thiazole) copolymer dye: spectroscopy, cyclic voltammetry and thermal analysis. Dyes Pigments 102:189–195. https://doi.org/10.1016/j.dyepig.2013.10.049
Jung G-Y, Choi JH, Lee JK (2015) Thermal behavior and ion conductivity of polyethylene oxide/polyhedral oligomeric silsesquioxane nanocomposite electrolytes. Adv Polym Technol 34. https://doi.org/10.1002/adv.21499
Liang X, Yang Y, Jin X et al (2015) The high performances of SiO2/Al2O3-coated electrospun polyimide fibrous separator for lithium-ion battery. J Membr Sci 493:1–7. https://doi.org/10.1016/j.memsci.2015.06.016
Ni’Mah YL, Cheng MY, Cheng JH et al (2015) Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J Power Sources 278:375–381. https://doi.org/10.1016/j.jpowsour.2014.11.047
Pitawala HMJC, Dissanayake MAKL, Seneviratne VA et al (2008) Effect of plasticizers (EC or PC) on the ionic conductivity and thermal properties of the (PEO)9LiTf: Al2O3 nanocomposite polymer electrolyte system. J Solid State Electrochem 12:783–789. https://doi.org/10.1007/s10008-008-0505-7
Niedzicki L, Kasprzyk M, Kuziak K et al (2009) Modern generation of polymer electrolytes based on lithium conductive imidazole salts. J Power Sources 192:612–617. https://doi.org/10.1016/j.jpowsour.2009.03.050
Wang H, Im D, Lee DJ et al (2013) A composite polymer electrolyte protect layer between lithium and water stable ceramics for aqueous lithium-air batteries. J Electrochem Soc 160:A728–A733. https://doi.org/10.1149/2.020306jes
Li Y, Zhan H, Wu L et al (2006) Flame-retarding ability and electrochemical performance of PEO-based polymer electrolyte with middle MW cyclic phosphate. Solid State Ionics 177:1179–1183. https://doi.org/10.1016/j.ssi.2006.05.015
Das S, Ghosh A (2017) Charge carrier relaxation in different plasticized PEO/PVDF-HFP blend solid polymer electrolytes. J Phys Chem B 121:5422–5432. https://doi.org/10.1021/acs.jpcb.7b02277
Karmakar A, Ghosh A (2010) A comparison of ion transport in different polyethylene oxide–lithium salt composite electrolytes. J Appl Phys 107:104113. https://doi.org/10.1063/1.3428389
Molinari N, Mailoa JP, Kozinsky B (2018) Effect of salt concentration on ion clustering and transport in polymer solid electrolytes: a molecular dynamics study of PEO–LiTFSI. Chem Mater. https://doi.org/10.1021/acs.chemmater.8b01955
Das S, Ghosh A (2015) Effect of plasticizers on ionic conductivity and dielectric relaxation of PEO-LiClO4 polymer electrolyte. Electrochim Acta 171:59–65. https://doi.org/10.1016/j.electacta.2015.04.178
Polu AR, Rhee H-W (2017) Ionic liquid doped PEO-based solid polymer electrolytes for lithium-ion polymer batteries. Int J Hydrog Energy 42:7212–7219. https://doi.org/10.1016/j.ijhydene.2016.04.160
Chaurasia SK, Shalu S, Gupta AK et al (2015) Role of ionic liquid [BMIMPF6] in modifying the crystallization kinetics behavior of the polymer electrolyte PEO-LiClO4. RSC Adv 5:8263–8277. https://doi.org/10.1039/C4RA12951B
Ramesh S, Liew C-W (2012) Tailor-made fumed silica-based nano-composite polymer electrolytes consisting of BmImTFSI ionic liquid. Iran Polym J 21:273–281. https://doi.org/10.1007/s13726-012-0022-5
Chakrabarti A, Filler R, Mandal BK (2008) Borate ester plasticizer for PEO-based solid polymer electrolytes. J Solid State Electrochem 12:269–272. https://doi.org/10.1007/s10008-007-0388-z
Henderson WA (2007) Crystallization kinetics of glyme - LiX and PEO - LiX polymer electrolytes. Macromolecules 40:4963–4971. https://doi.org/10.1021/ma061866d
Bernhard R, Latini A, Panero S et al (2013) Poly(ethylenglycol)dimethylether-lithium bis(trifluoromethanesulfonyl) imide, PEG500DME-LiTFSI, as high viscosity electrolyte for lithium ion batteries. J Power Sources 226:329–333. https://doi.org/10.1016/j.jpowsour.2012.10.059
Pradhan DK, Choudhary RNP, Samantaray BK et al (2007) Effect of plasticizer on structural and electrical properties of nanocomposite solid polymer electrolytes. Int J Electrochem Sci 2:861–871. https://doi.org/10.1007/s11581-010-0491-5
Vignarooban K, Dissanayake MAKL, Albinsson I, Mellander B-E (2014) Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide) (PEO) based solid polymer electrolytes. Solid State Ionics 266:25–28. https://doi.org/10.1016/j.ssi.2014.08.002
Zhao Y, Wu C, Peng G et al (2016) A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries. J Power Sources 301:47–53. https://doi.org/10.1016/j.jpowsour.2015.09.111
Klongkan S, Pumchusak J (2015) Effects of nano alumina and plasticizers on morphology, ionic conductivity, thermal and mechanical properties of PEO-LiCF3SO3 solid polymer electrolyte. Electrochim Acta 161:171–176. https://doi.org/10.1016/j.electacta.2015.02.074
Gitelman L, Israeli M, Averbuch A et al (2007) Modeling and simulation of Li-ion conduction in poly(ethylene oxide). J Comput Phys 227:1162–1175. https://doi.org/10.1016/j.jcp.2007.08.033
Zardalidis G, Ioannou E, Pispas S, Floudas G (2013) Relating structure, viscoelasticity, and local mobility to conductivity in PEO/LiTf electrolytes. Macromolecules 46:2705–2714. https://doi.org/10.1021/ma400266w
Stephan AM (2006) Review on gel polymer electrolytes for lithium batteries. Eur Polym J 42:21–42. https://doi.org/10.1016/j.eurpolymj.2005.09.017
Sharma P, Kanchan DK, Gondaliya N et al (2013) Conductivity relaxation in Ag+ ion conducting PEO–PMMA–PEG polymer blends. Ionics (Kiel) 19:301–307. https://doi.org/10.1007/s11581-012-0738-4
Sengwa RJ, Kaur K, Chaudhary R (2000) Dielectric properties of low molecular weight poly (ethylene glycol) s. Polym Int 49:599–608. https://doi.org/10.1002/1097-0126(200006)49:6<599::AID-PI425>3.0.CO;2-K
Sengwa RJ, Dhatarwal P, Choudhary S (2014) Role of preparation methods on the structural and dielectric properties of plasticized polymer blend electrolytes: correlation between ionic conductivity and dielectric parameters. Electrochim Acta 142:359–370. https://doi.org/10.1016/j.electacta.2014.07.120
Sengwa RJ, Dhatarwal P, Choudhary S (2015) Effects of plasticizer and nanofiller on the dielectric dispersion and relaxation behaviour of polymer blend based solid polymer electrolytes. Curr Appl Phys 15:135–143. https://doi.org/10.1016/j.cap.2014.12.003
Ali AMM, Subban RHY, Bahron H et al (2013) Investigation on modified natural rubber gel polymer electrolytes for lithium polymer battery. J Power Sources 244:636–640. https://doi.org/10.1016/j.jpowsour.2013.01.002
Yue L, Ma J, Zhang J et al (2016) All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater 5:139–164. https://doi.org/10.1016/j.ensm.2016.07.003
Ueno M, Imanishi N, Hanai K et al (2011) Electrochemical properties of cross-linked polymer electrolyte by electron beam irradiation and application to lithium ion batteries. J Power Sources 196:4756–4761. https://doi.org/10.1016/j.jpowsour.2011.01.054
Elizabeth RN, Kalyanasundaram S, Saito Y, Stephan AM (2005) Compatibility and thermal stability studies on plasticized PVC/PMMA blend polymer electrolytes complexed with different lithium salts. Polímeros 15:46–52. https://doi.org/10.1590/S0104-14282005000100011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Q., Chakrabarti, A., Mei, X. et al. New oligoether plasticizers for poly(ethylene oxide)-based solid polymer electrolytes. Ionics 25, 1633–1643 (2019). https://doi.org/10.1007/s11581-018-2752-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2752-7