Abstract
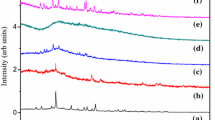
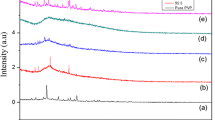
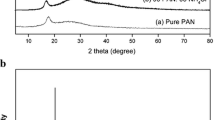
Poly (vinyl alcohol) (PVA) complexed with ammonium chloride (NH4Cl) solid polymer electrolyte films was prepared using solution casting technique. Complexation of dopant with polymer was studied using Fourier transform infrared spectroscopy (FTIR). X-ray diffraction (XRD) results reflect that degree of crystallinity of the polymer increases with doping level. Surface morphology and topology of the electrolyte were studied using Scanning electron microscopy (SEM) and atomic force microscopy (AFM). Thermogravimetric analysis (TGA) results accounts for the increase of thermal stability of PVA due to the incorporation of NH4Cl. Impedance analysis was carried out to understand relaxation phenomena of polymer electrolyte. Dielectric studies revealed that the mobility of charge carriers follows non-Debye nature of ionic relaxation. Electrical studies reveal that proton conduction occurs in this polymer electrolyte through Grotthus mechanism and maximum conductivity of the polymer electrolyte (7.5 wt% NH4Cl/PVA) has been observed to be 1.81 × 10−3 S/cm at 353 K. Transport parameters have been determined using Wagner’s polarization technique. The observed highest conductivity of polymer electrolyte indicates utilizing this electrolyte for the fabrication of proton battery applications, and accordingly, the cell parameters have been measured.










Similar content being viewed by others
References
Kumar A, Logapperumal S, Sharma R, Das MK, Kar KK (2016) Li-ion transport, structural and thermal studies on lithium triflate and barium titanate incorporated poly (vinylidene fluoride-co-hexafluoropropene) based polymer electrolyte. Solid State Ionics 289:150–158. https://doi.org/10.1016/j.ssi.2016.03.008
Naga G, Vani S, Raja V, Sharma AK, Rao VVRN (2013) Development of PVP based polymer electrolytes for solid state battery applications. Int J Res Eng Adv Technol 1(3) ISSN: 2320–8791. https://www.researchgate.net/publication/317003139
Chew KW, Ng TC, How ZH (2013) Conductivity and microstructure study of PLA-based polymer electrolyte salted with Lithium Perchloride, LiClO4. Int J Electrochem Sci 8:6354–6364
Bamford D, Dlubek G, Reiche A, Alam MA, Meyer W, Galvosas P, Rittig F (2001) The local free volume, glass transition, and ionic conductivity in a polymer electrolyte: a positron lifetime study. J Chem Phys 115:7260. https://doi.org/10.1063/1.1402633
Rana P, Singh B, Chandra S (2006) Polymeric rechargeable solid-state proton battery. J Power Sources 161:702–706. https://doi.org/10.1016/j.jpowsour.2006.04.020
Agrawal RC, Hashmi SA, Pandey GP (2007) Electrochemical cell performance studies on all-solid-state battery using nano-composite polymer electrolyte membrane. Ionics 13:295–298. https://doi.org/10.1007/s11581-007-0112-0
Hema M, Selvasekerapandian S, Hirankumar G (2009) A. Sakunthala, D. Arunkumar, H. Nithya, structural and thermal studies of PVA:NH4I. J Phys Chem Solids 70:1098–1103. https://doi.org/10.1016/j.jpcs.2009.06.005
Ahmad NH, Isa MIN (2015) Structural and ionic conductivity studies of CMC based polymer electrolyte doped with NH4Cl. Adv Mater Res ISSN: 1662-8985 1107:247–252. https://doi.org/10.4028/www.scientific.net/AMR.1107.247
Radha KP, Selvasekarapandian S, Karthikeyan S, Hema M, Sanjeeviraja C (2013) Synthesis and impedance analysis of proton-conducting polymer electrolyte PVA:NH4F. Ionics 19:1437–1447. https://doi.org/10.1007/s11581-013-0866-5
Manjunath A, Deepa T, Supreetha NK, Irfan M (2015) Studies on AC electrical conductivity and dielectric properties of PVA/NH4NO3 solid polymer electrolyte films. Adv Mater Phys Chem 5:295–301. https://doi.org/10.4236/ampc.2015.58029
Praveena SD, Ravindrachary V, Bhajantri RF, Ismayil (2016) Dopant-induced microstructural, optical, and electrical properties of TiO2/PVA composite. Polym Compos 37:987–997. https://doi.org/10.1002/pc.23258
Vinoth Pandi D, Selvasekarapandian S, Bhuvaneswari R, Premalatha M, Monisha S, Arunkumar D, Junichi K (2016) Development and characterization of proton conducting polymer electrolyte based on PVA, amino acid glycine and NH4SCN. Solid State Ionics 298:15–22. https://doi.org/10.1016/j.ssi.2016.10.016
Sikkanthar S, Karthikeyan S, Selvasekarapandian S, Arunkumar D, Nithya H, Junichi K (2016) Structural, electrical conductivity, and transport analysis of PAN–NH4Cl polymer electrolyte system. Ionics 22:1085–1094. https://doi.org/10.1007/s11581-016-1645-x
Bhajantri RF, Ravindrachary V, Harisha A, Crasta V, Nayak SP, Poojary B (2006) Microstructural studies on BaCl2 doped poly (vinyl alcohol). Polymer 47:3591–3598. https://doi.org/10.1016/j.polymer.2006.03.054
Hari-Bala, Guo Y, Xu Z, Zhao J, Wuyou F, Ding X, Jiang Y, Yu K, Lv X, Wang Z (2006) Controlling the particle size of nanocrystalline titania via a thermal dissociation of substrates with ammonium chloride. Mater Lett 60:494–498. https://doi.org/10.1016/j.matlet.2005.09.030
Fernandes DM, Aiation A, Winkler H, Job AE, Radovanocic E, Gomez Pineda EA (2006) Thermal and photochemical stability of poly (vinyl alcohol)/modified lignin blends. Polym Degrad Stab 91:1192–1201. https://doi.org/10.1016/j.polymdegradstab.2005.05.024
Kesavana K, Mathewa CM, Rajendrana S, Ulaganathan M (2014) Preparation and characterization of novel solid polymer blend electrolytes based on poly (vinyl pyrrolidone) with various concentrations of lithium perchlorate. Mater Sci Eng B 184:26–33. https://doi.org/10.1016/j.mseb.2014.01.009
Ramaraj B, Jaisankar SN (2008) Thermal and morphological properties of poly (vinyl alcohol) and layered double hydroxide (LDH) Nanocomposites. Polym-Plast Technol Eng 47:733–738. https://doi.org/10.1080/03602550802059170
G. Attia, M.F.H. Abd El-kader, Structural (2013) Optical and thermal characterization of PVA/2HEC Polyblend films. Int J Electrochem Sci 8:5672–5687
Abdelrazek EM, Elashmawi IS (2008) Characterization and physical properties of CoCl2 filled polyethyl methacrylate films. Polym Compos 29:1036–1043. https://doi.org/10.1002/pc.20481
Coats AW, Redfern JP (1984) Kinetic parameters from Thermogravimetric data. Nature 201:681. https://doi.org/10.1038/201068a0
Abdelaziz M (2011) Cerium (III) doping effects on optical and thermal properties of PVA films. Physica B 406:1300–1307. https://doi.org/10.1016/j.physb.2011.01.021
Sai Prasanna CM, Austin Suthanthiraraj S (2017) Dielectric, thermal, and electrochemical properties of PVC/PEMA blended polymer electrolytes complexed with zinc triflate salt. Ionics 23:3137–3150. https://doi.org/10.1007/s11581-017-2109-7
Kalim D, Basheer Ahamed M, Polu AR, Sadasivuni KK, Khadheer Pasha SK, Ponnamma D, AlMaadeed MA-A, Deshmukh RR, Chidambaram K (2016) Impedance spectroscopy, ionic conductivity and dielectric studies of new li+ ion conducting polymer blend electrolytes based on biodegradable polymers for solid state battery applications. J Mater Sci Mater Electron 27:11410–11424. https://doi.org/10.1007/s10854-016-5267-x
Vidyashree H, Bhajantri RF, Naik J (2017) Physico-chemical properties of bismuth nitrate filled PVA–LiClO4 polymer composites for energy storage application. J Mater Sci Mater Electron 28:5827–5839. https://doi.org/10.1007/s10854-016-6254-y
Shreedatta H, Ravindrachary V, Praveena SD, Guruswamy B, Sagar RN (2018) Optical and Dielectric Properties of Li+ ion conducting solid polymer electrolyte. Ind J Adv Chem Sci 6(2):83–87. https://doi.org/10.22607/IJACS.2018.602004
Mahendia S, Goyal PK, Tomar AK, Chahal RP, Kumar S (2016) Study of dielectric behavior and charge conduction mechanism of poly(Vinyl Alcohol) (PVA)-Copper (Cu) and gold (Au) nanocomposites as a bio-resorbable material for organic electronics. J Electron Mater 45(10). https://doi.org/10.1007/s11664-016-4677-0
Ragab HM (2011) Spectroscopic investigations and electrical properties of PVA/PVP blend filled with different concentrations of nickel chloride. Physica B 406:3759–3767. https://doi.org/10.1016/j.physb.2010.11.030
Buraidah MH, Teo LP, Majid SR, Arof AK (2009) Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Physica B 404:1373–1379. https://doi.org/10.1016/j.physb.2008.12.027
Tahalyania J, Rahangdalea KK, Aepurua R, Balasubramanian K, Datar S (2016) Dielectric investigation of conducting fibrous nonwoven porous mat fabricated by one-step facile electrospinning process. RSC Adv 6:36588. https://doi.org/10.1039/C5RA23012H
Ben Taher Y, Oueslati A, Maaloul NK, Khirouni K, Gargouri M (2015) Conductivity study and correlated barrier hopping (CBH) conduction mechanism in diphosphate compound. Appl Phys A Mater Sci Process 120:1537–1543. https://doi.org/10.1007/s00339-015-9353-3
Rathod SG, Bhajantri RF, Ravindrachary V, Naik J, Madhu Kumar DJ (2016) High mechanical and pressure sensitive dielectric properties of Graphene oxide doped PVA Nanocomposites. RSC Adv 6:77977–77986. https://doi.org/10.1039/C6RA16026C
Bi L, Shafi SP, Da’as EH, Traversa E (2018) Tailoring the cathode–electrolyte Interface with nanoparticles for boosting the solid oxide fuel cell performance of chemically stable proton-conducting electrolytes. Small 14:1801231. https://doi.org/10.1002/smll.201801231
Ma J, Tao Z, Kou H, Fronzi M, Bi L (2019) Evaluating the effect of Pr-doping on the performance of strontium-doped lanthanum ferrite cathodes for protonic SOFCs. Ceram Int. https://doi.org/10.1016/j.ceramint.2019.10.017
Xu X, Wang H, Jinming M, Liu W, Wang X, Fronzi M, Bi L (2019) Impressive performance of proton-conducting solid oxide fuel cells using a first-generation cathode with tailored cations. J Mater Chem A 7:18792–18798. https://doi.org/10.1039/C9TA06676D
Xia Y, Jin Z, Wang H, Zheng G, Lv H, Peng R, Liu W, Bi L (2019) A novel cobalt-free cathode with triple-conduction for proton-conducting solid oxide fuel cells with unprecedented performance. J Mater Chem A 7:16136–16148. https://doi.org/10.1039/c9ta02449b
Xu X, Lei B, Zhao XS (2018) Highly-conductive proton-conducting electrolyte membranes with a low sintering temperature for solid oxide fuel cells. J Membr Sci 558:17–25. https://doi.org/10.1016/j.memsci.2018.04.037
Krishna Kumar V, Shanmugam G (2012) Electrical and optical properties of pure and Pb2+ ion doped PVA−PEG polymer composite electrolyte films. Ionics 18:403–411. https://doi.org/10.1007/s11581-011-0643-2
Du JF, Bai Y, Chu WY, Qiao LJ (2010) Synthesis and performance of proton ConductingChitosan/NH4Cl electrolyte. J Polym Sci B Polym Phys 48:260–266. https://doi.org/10.1002/polb.21866
Shreedatta H, Ravindrachary V, Praveena SD, Guruswamy B, Sagar RN, Sanjeev G (2018) Relaxation and transport properties of li+ conducting biocompatible material for battery application. Ion AIP Conf Proc 1942:110043. https://doi.org/10.1063/1.5029026
Nadimicherla R, Sharma AK, Narasimha Rao VVR, Chen W (2014) AC conduction mechanism and battery discharge characteristics of (PVC/PEO) polyblend films complexed with potassium chloride. Measurement 47:33–41. https://doi.org/10.1016/j.measurement.2013.08.047
Hemalatha R, Alagar M, Selvasekarapandian S, Sundaresan B, Moniha V, Boopathi G, Christopher Selvin P (2019) Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acidproline, and NH4Cl and its applications to electrochemical devices. Ionics 25:141–154. https://doi.org/10.1007/s11581-018-2564-9
Kathayat K, Panigrahi A, Pandey A, Kar S (2012) Characterization of electrical behavior of Ba5HoTi3V7O30 ceramic using impedance analysis. Mater Sci Appl 3:390–397. https://doi.org/10.4236/msa.2012.36056
Chawla M, Shekhawat N, Aggarwa S, Sharma A, Nair KGM (2014) Cole-cole analysis and electrical conduction mechanism of N+ implanted polycarbonate. J Appl Phys 115:184104. https://doi.org/10.1063/1.4876123
Saroj AL, Singh RK (2012) Thermal, dielectric and conductivity studies on PVA/ionic liquid [EMIM][EtSO4] based polymer electrolytes. J Phys Chem Solids 73:162–168. https://doi.org/10.1016/j.jpcs.2011.11.012
Sahoo PS, Panigrahi A, Patri SK, Choudhary RNP (2009) Structural and impedance properties of Ba5 DyTi3V7O30. J Mater Sci Mater Electron 20:565–570. https://doi.org/10.1007/s10854-008-9766-2
Wagner B, Wagner C (1957) Electrical conductivity measurements on cuprous halides. J Chem Phys 26:1597. https://doi.org/10.1063/1.1743590
Prajapati GK, Roshan R, Gupta PN (2010) Effect of plasticizer on ionic transport and dielectric properties of PVA–H3PO4 proton conducting polymeric electrolytes. J Phys Chem Solids 71:1717–1723. https://doi.org/10.1016/j.jpcs.2010.08.023
Bhavani S, Ravi M, Rao VVRN (2014) Studies on electrical properties of PVA: NiBr2 complexed polymer electrolyte films for battery applications. Int J Eng Sci Innov Technol 3(4) ISSN: 2319–5967
Anantha PS, Hariharan K (2005) Physical and ionic transport studies on poly (ethylene oxide)–NaNO3 polymer electrolyte system. Solid State Ionics 176:155–162. https://doi.org/10.1016/j.ssi.2004.07.006
Leena Chandra MV, Karthikeyan S, Selvasekarapandian S, Vinoth Pandi D, Monisha S, Arulmozhi Packiaseeli S (2016) Characterization of high ionic conducting PVAc–PMMA blend-based polymer electrolyte for electrochemical applications. Ionics 22:2409–2420. https://doi.org/10.1007/s11581-016-1763-5
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Sanjeeviraja C, Nithya H, Iwai Y, Kawamura J (2015) Proton-conducting polymer electrolyte based on PVA-PAN blend doped with ammonium thiocyanate. Ionics 21:1017–1029. https://doi.org/10.1007/s11581-014-1259-0
Acknowledgements
The authors are thankful to Prof. Ganesh Sanjeev, Microtron Center, Mangalore University, for providing instruments to carry out electrical studies and PURSE Lab Mangalore University for providing SEM and TGA facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hegde, S., Ravindrachary, V., Praveena, S.D. et al. Microstructural, dielectric, and transport properties of proton-conducting solid polymer electrolyte for battery applications. Ionics 26, 2379–2394 (2020). https://doi.org/10.1007/s11581-019-03383-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03383-w