Abstract
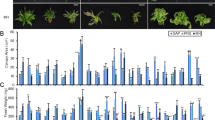
Micropropagation is a preferred method to propagate clean, clonal stock plants. Subculture is labor intensive and costly. In vitro hedging can reduce hood labor and was demonstrated with cannabis (Cannabis sativa L. ‘US Nursery Cherry 1’) using both apical and nodal explants at four different photosynthetic photon flux densities (25–167 μmol m−2 s−1) in vessels with vented or non-vented closures. The numbers of harvested shoot tips from four repeated 3-wk cutting cycles without subculture and the quality of the harvested shoot tips during ex vitro rooting in phenolic foam plugs were observed. The number of shoot tips harvested in non-vented vessels increased over four repeated cycles; however, there was a decline in number of shoot tips harvested from vented vessels in the fourth cycle due to excessive drying and collapse of the agar matric. The number of shoot tips harvested through repeated cycles was increased with light intensity. Nearly 100% of the micro-cuttings from optimal light and ventilation rooted ex vitro. Most plantlets had roots that penetrated the exterior surfaces of the plug after 2 wk. The number of leaves per rooted plantlet ex vitro increased with light intensity in vitro. Micropropagation labor efficiency could be improved by using a multi-cycle cutting process which would allow the same material to be repeatedly cut from rooted bases instead of subcultured. In a system with strong apical dominance, enhanced axillary divisions of shoot tip explants over successive cycles of cutting, without the need of exogenous PGRs, were further increased by appropriate in vitro factors, light, and ventilation, presenting labor saving potential compared to standard, single-cut system in common use.







Similar content being viewed by others
References
Adelberg J, Kroggel M, Toler J (2000) Physical environment in vitro affects laboratory and nursery growth of micropropagated Hostas. HortTechnology 10:754–757
Ahloowalia BS, Savangikar VA (2004) Low cost options for energy and labour. In: Low cost options for tissue culture technology in developing countries. IAEA-TECDOC-1384 pp. 41–45. International Atomic Energy Agency, Vienna, Austria
Aitken-Christie J, Davies HE (1988) Development of a semi-automated micropropagation system. Acta Hort. 230:81–87
Aitken-Christie J, Jones C (1987) Towards automation: radiata pine shoot hedges in vitro. Plant Cell Tiss Org Cult 8:185–196
Chandra S, Lata H, El Sohly MA (Eds.). Cannabis sativa L.-botany and biotechnology In: Cannabis sativa L.—botany and biotechnology. Springer, Cham, pp. 79–100(2017)
Chaohua C, Gonggu Z, Lining Z, Chunsheng G, Qing T, Jianhua C, Xinbo G, Dingxiang P, Jianguang S (2016) A rapid shoot regeneration protocol from the cotyledons of hemp (Cannabis sativa L.). Ind Crop Prod 83:61–65
Debergh PC, Topoonyanont N, Van Huylenbroeck J, Moreira da Silva H, Oyaert E (2000) Preparation of microplants for ex vitro establishment. Acta Hort. 530:269–275
Desjardins Y (1994) Photosynthesis in vitro-on the factors regulating CO2 assimilation in micropropagation systems. Env Eff Con Plant Tiss Cult 393:45–62
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. HortScience 19:507–509
Economou AS (2011) From microcutting rooting to microplant establishment: key points to consider for maximum success in woody plants. Acta Hort 988:43–56
El-Hawaz RF, Adelberg J, Naylor-Adelberg J, Eisenreich R, Van der Meij J (2019) The effect of slow-growth strategy on a production of Petunia× hybrida Vilm. microcuttings. In Vitro Cell Dev Biol - Plant 55:433–441
ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A (2017) Phytochemistry of Cannabis sativa L. In: Phytocannabinoids. Springer, Cham, pp 1–36
Fujiwara K, Kozai T (1995) Physical microenvironment and its effects. In: Aitken-Cristie J, Kozai T, Smith MAL (eds) Automation and Environmental Control in Plant Tissue Culture. Kluwer Academic Publishers, Dordrecht, pp 319–369
Hazarika BN (2003) Acclimatization of tissue-cultured plants. Curr Sci 85:1704–1712
Hoagland DR, Snyder WC (1933) Nutrition of strawberry plants under controlled conditions. AmerSoc Hort Sci 30:288–294
Kitaya Y, Fukuda O, Kozai T, Kirdmanee C (1995) Effects of light intensity and lighting direction on the photoautotrophic growth and morphology of potato plantlets in vitro. Sci Hortic 62:15–24
Kozai T, Iwanami Y (1988) Effects of CO2 enrichment and sucrose concentration under high photon fluxes on plantlet growth of carnation (Dianthus caryophyllus L.) in tissue culture during the preparation stage. J Japanese Soc Hort Sci 57:279–288
Kozai T, Koyama Y, Watanabe I (1988) Multiplication and rooting of potato plantlets in vitro with sugar-free medium under high photosynthetic photon flux Acta Hort 230:121–127
Lata H, Chandra S, Khan I, ElSohly MA (2009) Thidiazuron-induced high-frequency direct shoot organogenesis of Cannabis sativa L. In Vitro Cell Dev Biol - Plant 45:12–19
Lata H, Chandra S, Khan IA, ElSohly MA (2017) Micropropagation of Cannabis sativa L.—an update. In: Chandra S, Lata H, El Sohly M (eds) Cannabis sativa L. - Botany and Biotechnology. Springer, Cham, pp 285–297
Lata H, Chandra S, Techen N, Khan IA, ElSohly MA (2016) In vitro mass propagation of Cannabis sativa L.: a protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J Appl Res Med Aromat Plant 3:18–26
Lee TJ, Zobayed SMA, Firmani F, Park EJ (2019) A novel automated transplanting system for plant tissue culture. Biosyst Eng 181:63–72
Mestinšek Mubi Š, Svetik S, Flajšman M, Murovec J (2020) In vitro tissue culture and genetic analysis of two high-CBD medical cannabis (Cannabis sativa L.) breeding lines. Genetika 52(3):925–941
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Neto AR, Chagas EA, Costa BNS, Chagas PC, Vendrame WA (2020) Photomixotrophic growth response of sugarcane in vitro plantlets using different light intensities and culture vessel types. In Vitro Cell Dev Biol-Plant 56:504–514
Page SR, Monthony AS, Jones AMP (2020) Basal media optimization for the micropropagation and callogenesis of Cannabis sativa L. BioRxiv 1–23
Piunno KF, Golenia G, Boudko EA, Downey C, Jones AMP (2019) Regeneration of shoots from immature and mature inflorescences of Cannabis sativa. Can J Plant Sci 99:556–559
Rohr R, Iliev I, Scaltsoyinnes A, Tsoulpha P (2001) Acclimatization of micropropagated forest trees. Acta Hort 616:59–69
da Silva JAT, Dobránszki J, Ross S (2013) Phloroglucinol in plant tissue culture. In Vitro Cell Dev Biol - Plant 49:1–16
Sluis CJ (2008) Integrating automation technologies with commercial micropropagation. In: Plant Tissue Culture Engineering. Springer, Dordrecht, pp 231–251
Smith M, Palta J, McCown B (1986) Comparative anatomy and physiology of microcultured, seedling, and greenhouse-grown Asian white birch. J Amer Soc Hort Sci 111:437–442
Van Staden J, Fennell CW, Taylor NJ (2006) Plant stress in vitro: the role of phytohormones. Acta Hort 725:55–61
Wang R, He LS, Xia B, Tong JF, Li N, Peng F (2009) A micropropagation system for cloning of hemp (Cannabis sativa L.) by shoot tip culture. Pak J Bot 41:603–608
Wetzstein H, Sommer H (1982) Leaf anatomy of tissue cultured Liquidambar styraciflua (Hamamelidaceae) during acclimatization. Amer J Bot 69:1579–1586
Wróbel T, Dreger M, Wielgus K, Słomski R (2020) Modified nodal cuttings and shoot tips protocol for rapid regeneration of Cannabis sativa L. J Natl Fibers 19:1–10
Ziv M (1995) In vitro acclimatization. In: Aitken-Cristie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Kluwer Academic Publishers, Dordrecht, pp 493–516
Zobayed SMA (2005) Ventilation in micropropagation. In: Photoautotrophic (sugar-free medium) micropropagation as a new micropropagation and transplant production system. Springer, Dordrecht, pp 147–186
Zobayed SMA, Armstrong J, Armstrong W (2001) Micropropagation of potato: evaluation of closed, diffusive and forced ventilation on growth and tuberization. Ann Bot 87:53–59
Funding
This research was supported by a grant from the South Carolina Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Mr. Murphy reports grants from South Carolina Department of Agriculture, during the conduct of the study; In addition, Dr. Adelberg has a patent Multicutter system for plant micropropagation pending.
Dr. Adelberg reports grants from SC Department of Agriculture, during the conduct of the study.
Additional information
Editor: Pamela Weathers
Rights and permissions
About this article
Cite this article
Murphy, R., Adelberg, J. Physical factors increased quantity and quality of micropropagated shoots of Cannabis sativa L. in a repeated harvest system with ex vitro rooting. In Vitro Cell.Dev.Biol.-Plant 57, 923–931 (2021). https://doi.org/10.1007/s11627-021-10166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-021-10166-4