Abstract
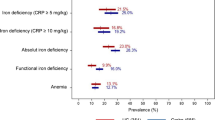
Iron deficiency anemia (IDA) is one of the most common complications of inflammatory bowel disease (IBD). We planned a prospective study to address tolerability and efficacy of sucrosomial iron, a new oral formulation of ferric pyrophosphate, in IBD patients. Thirty patients with a confirmed diagnosis of Crohn’s Disease (CD) or ulcerative colitis (UC) and mild IDA were enrolled. Patients with severe IBD were excluded. All patients underwent 12 weeks of oral treatment with 30 mg/day of sucrosomial iron. Treatment compliance and adverse events were investigated every 4 weeks. Iron status, hematological parameters and IBD activity scores were determined at baseline and at the end of treatment, as well as serum hepcidin and non-transferrin bound iron (NTBI) levels. Twenty-four (80%) patients took more than 90% of the prescribed regimen. Forty-four adverse events (AEs) were recorded, but none of them is considered certainly or probably related to the study treatment. Interestingly, only eleven gastrointestinal events were recorded in 9 (30%) patients. At the end of treatment, all iron parameters improved significantly and Hb increased in 86% of patients (from 11.67 to 12.37 g/dl, p = 0.001). Serum hepcidin showed a significant increase in 79% of patients and became positively correlated with C-reactive protein (CRP) at the end of the study, while NTBI remained below the detection threshold after iron supplementation. The IBD activity scores improved in both CD and UC. This pilot interventional study supports the therapeutic use of sucrosomial iron in IBD and paves the way for future studies in larger or more difficult IBD populations.

Similar content being viewed by others
References
Testa A, Rispo A, Romano M, Riegler G, Selvaggi F, Bottiglieri E, Martorano M, Rea M, Gravina A, Nardone OM, Patturelli M, Pellino G, Miranda A, Caporaso N, Castiglione F (2016) The burden of anaemia in patients with inflammatory bowel diseases. Dig Liver Dis 48:267–270. https://doi.org/10.1016/j.dld.2015.10.012
Filmann N, Rey J, Schneeweiss S, Ardizzone S, Bager P, Bergamaschi G, Koutroubakis I, Lindgren S, Morena Fde L, Moum B, Vavricka SR, Schroder O, Herrmann E, Blumenstein I (2014) Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis 20:936–945. https://doi.org/10.1097/01.MIB.0000442728.74340.fd
Bergamaschi G, Di Sabatino A, Albertini R, Ardizzone S, Biancheri P, Bonetti E, Cassinotti A, Cazzola P, Markopoulos K, Massari A, Rosti V, Porro GB, Corazza GR (2010) Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica 95:199–205. https://doi.org/10.3324/haematol.2009.009985
Kulnigg S, Gasche C (2006) Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther 24:1507–1523. https://doi.org/10.1111/j.1365-2036.2006.03146.x
Zimmermann MB, Hurrell RF (2007) Nutritional iron deficiency. Lancet 370:511–520. https://doi.org/10.1016/S0140-6736(07)61235-5
Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S, Oldenburg B, Rampton D, Schroeder O, Stein J, Travis S, Van Assche G (2007) Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis 13:1545–1553. https://doi.org/10.1002/ibd.20285
Theurl I, Schroll A, Nairz M, Seifert M, Theurl M, Sonnweber T, Kulaksiz H, Weiss G (2011) Pathways for the regulation of hepcidin expression in anemia of chronic disease and iron deficiency anemia in vivo. Haematologica 96:1761–1769. https://doi.org/10.3324/haematol.2011.048926
Dignass AU, Gasche C, Bettenworth D, Birgegard G, Danese S, Gisbert JP, Gomollon F, Iqbal T, Katsanos K, Koutroubakis I, Magro F, Savoye G, Stein J, Vavricka S, Cs European, Colitis O (2015) European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 9:211–222. https://doi.org/10.1093/ecco-jcc/jju009
Kaitha S, Bashir M, Ali T (2015) Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol 6:62–72. https://doi.org/10.4291/wjgp.v6.i3.62
Lee TW, Kolber MR, Fedorak RN, van Zanten SV (2012) Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis 6:267–275. https://doi.org/10.1016/j.crohns.2011.09.010
de Silva AD, Tsironi E, Feakins RM, Rampton DS (2005) Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther 22:1097–1105. https://doi.org/10.1111/j.1365-2036.2005.02700.x
Grisham MB (1994) Oxidants and free radicals in inflammatory bowel disease. Lancet 344:859–861
Erichsen K, Ulvik RJ, Grimstad T, Berstad A, Berge RK, Hausken T (2005) Effects of ferrous sulphate and non-ionic iron-polymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Aliment Pharmacol Ther 22:831–838. https://doi.org/10.1111/j.1365-2036.2005.02652.x
Lund EK, Wharf SG, Fairweather-Tait SJ, Johnson IT (1999) Oral ferrous sulfate supplements increase the free radical-generating capacity of feces from healthy volunteers. Am J Clin Nutr 69:250–255. https://doi.org/10.1093/ajcn/69.2.250
Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY (2002) Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci 47:1266–1278
Zimmermann MB, Chassard C, Rohner F, N’Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF (2010) The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr 92:1406–1415. https://doi.org/10.3945/ajcn.110.004564
Simao AMS, Bolean M, Cury TAC, Stabeli RG, Itri R, Ciancaglini P (2015) Liposomal systems as carriers for bioactive compounds. Biophys Rev 7:391–397. https://doi.org/10.1007/s12551-015-0180-8
Brilli E, Romano A, Fabiano A, Zambito Y, Di Raimondo F, Tarantino G (2016) Sucrosomial technology is able to promote ferric iron absorption: pre-clinical and clinical evidences. Blood 128:3618
Pisani A, Riccio E, Sabbatini M, Andreucci M, Del Rio A, Visciano B (2015) Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: a randomized trial. Nephrol Dial Transplant 30:645–652. https://doi.org/10.1093/ndt/gfu357
Gomollon F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P, Ecco (2017) 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 11:3–25. https://doi.org/10.1093/ecco-jcc/jjw168
Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagorowicz E, Raine T, Harbord M, Rieder F, Cs European, Colitis O (2017) Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 11:649–670. https://doi.org/10.1093/ecco-jcc/jjx008
Rachmilewitz D (1989) Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 298:82–86
Best WR, Becktel JM, Singleton JW, Kern F Jr (1976) Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 70:439–444
Guidelines for Good Clinical Practice E6(R1), (1996) International Conference on harmonization of technical requirements
Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P (2005) LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol 18:277–287. https://doi.org/10.1016/j.beha.2004.10.003
Zipperer E, Post JG, Herkert M, Kundgen A, Fox F, Haas R, Gattermann N, Germing U (2013) Serum hepcidin measured with an improved ELISA correlates with parameters of iron metabolism in patients with myelodysplastic syndrome. Ann Hematol 92:1617–1623. https://doi.org/10.1007/s00277-013-1839-5
Zanninelli G, Breuer W, Cabantchik ZI (2009) Daily labile plasma iron as an indicator of chelator activity in Thalassaemia major patients. Br J Haematol 147:744–751. https://doi.org/10.1111/j.1365-2141.2009.07907.x
Schmidt C, Ahmad T, Tulassay Z, Baumgart DC, Bokemeyer B, Howaldt S, Stallmach A, Buning C, Group AS (2016) Ferric maltol therapy for iron deficiency anaemia in patients with inflammatory bowel disease: long-term extension data from a Phase 3 study. Aliment Pharmacol Ther 44:259–270. https://doi.org/10.1111/apt.13665
Schroder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M, Stein J (2005) Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease—a randomized, controlled, open-label, multicenter study. Am J Gastroenterol 100:2503–2509. https://doi.org/10.1111/j.1572-0241.2005.00250.x
Pietrangelo A, Trautwein C (2004) Mechanisms of disease: the role of hepcidin in iron homeostasis—implications for hemochromatosis and other disorders. Nat Clin Pract Gastroenterol Hepatol 1:39–45. https://doi.org/10.1038/ncpgasthep0019
Ganz T, Nemeth E (2012) Hepcidin and iron homeostasis. Biochim Biophys Acta 1823:1434–1443. https://doi.org/10.1016/j.bbamcr.2012.01.014
Bergamaschi G, Di Sabatino A, Albertini R, Costanzo F, Guerci M, Masotti M, Pasini A, Massari A, Campostrini N, Corbella M, Girelli D, Corazza GR (2013) Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis 19:2166–2172. https://doi.org/10.1097/MIB.0b013e31829a6e43
Mecklenburg I, Reznik D, Fasler-Kan E, Drewe J, Beglinger C, Hruz P, Swiss IBDCSG (2014) Serum hepcidin concentrations correlate with ferritin in patients with inflammatory bowel disease. J Crohns Colitis 8:1392–1397. https://doi.org/10.1016/j.crohns.2014.04.008
Dresow B, Petersen D, Fischer R, Nielsen P (2008) Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals 21:273–276. https://doi.org/10.1007/s10534-007-9116-5
Schumann K, Kroll S, Weiss G, Frank J, Biesalski HK, Daniel H, Friel J, Solomons NW (2005) Monitoring of hematological, inflammatory and oxidative reactions to acute oral iron exposure in human volunteers: preliminary screening for selection of potentially-responsive biomarkers. Toxicology 212:10–23. https://doi.org/10.1016/j.tox.2005.03.014
Erichsen K, Milde AM, Arslan G, Helgeland L, Gudbrandsen OA, Ulvik RJ, Berge RK, Hausken T, Berstad A (2005) Low-dose oral ferrous fumarate aggravated intestinal inflammation in rats with DSS-induced colitis. Inflamm Bowel Dis 11:744–748
Tarantino G, Brilli E, Zambito Y, Giordano G, Equitani F (2015) Sucrosomial Iron®: a new highly bioavaible oral iron supplement. Blood 126:4561
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Abbati, G., Incerti, F., Boarini, C. et al. Safety and efficacy of sucrosomial iron in inflammatory bowel disease patients with iron deficiency anemia. Intern Emerg Med 14, 423–431 (2019). https://doi.org/10.1007/s11739-018-1993-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1993-9