Abstract
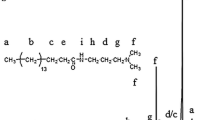
Concentration of the surface active agents in industrial products is a common source of error. In order to compare the efficiency of a number of polyisobutylene succinic anhydride (PIBSA) based surfactants, their concentration needs to be determined with a fair degree of accuracy. Industrial samples of the monoethanolamine adduct of PIBSA (PIBSA-MEA) concentrate were used for chromatographic separation of the functionalized surfactant from the sample matrix. Complete spectroscopic assignments were based on detailed analysis of all the precursors and of the purified mixture of structural isomers. The structures of the double bond isomers were consistent with the expected addition products of the classic Alder-ene reaction-derived PIBSA. The carbon–carbon connectivity of the succinamide head group to the bulky polymer tail of PIBSA-MEA was more complicated than previously thought, pointing towards regioselectivity in the nucleophilic substitution of PIBSA. By analogy, further structural assignments of two other surfactants, branded as PIBSA-IMIDE and PIBSA-UREA were made from the spectroscopic data recorded on crude industrial samples. Detailed nuclear magnetic resonance (NMR) assignments for all three surfactants reported here were utilized to develop a semi-quantitative 13C-NMR based method for the estimation of the amount of the functionalized surfactant relative to the total PIB content in the industrial concentrates. The results highlight common sources of structure- and concentration-dependent errors in high internal phase emulsion formulations.







Similar content being viewed by others
References
Forsberg JW. Water-in-oil emulsions. US Patent 4,708,753. 1987.
Chattopadhyay AK. Water-in-oil emulsion explosive. US Patent 4,919,179. 1990.
Ganguly S, Mohan VK, Bhasu VCJ, Mathews E, Adiseshaiah KS, Kumar AS. Surfactant–electrolyte interactions in concentrated water-in-oil emulsions: FT-IR spectroscopic and low-temperature differential scanning calorimetric studies. Coll Surf. 1992;65(4):243–256. doi:10.1016/0166-6622(92)80180-A.
Ghaicha L, Leblanc RM, Chattopadhyay AK. Influence of concentrated ammonium nitrate solution on monolayers of some dicarboxylic acid derivatives at the air/water interface. Langmuir. 1993;9(1):288–293. doi:10.1021/la00025a055.
Derkach SR. Rheology of emulsions. Adv Colloid Interface Sci. 2009;151(1–2):1–23. doi:10.1016/j.cis.2009.07.001.
Reynolds PA, McGillivray DJ, Mata JP, Yaron PN, White JW. The stability of high internal phase emulsions at low surfactant concentration studied by small angle neutron scattering. J Colloid Interface Sci. 2010;349(2):544–553. doi:10.1016/j.jcis.2010.05.082.
Babak VG, Stébé M-J. Highly concentrated emulsions: physicochemical principles of formulation. J Dispers Sci Technol. 2002;23(1–3):1–22. doi:10.1080/01932690208984184.
Reynolds PA, Gilbert EP, White JW. High internal phase water-in-oil emulsions and related microemulsions studied by small angle neutron scattering. 2. The distribution of surfactant. J Phys Chem B. 2001;105(29):6925–6932. doi:10.1021/jp010349o.
Boer WG. Composition and emulsifier. US Patent 6,630,596. 2003.
Reynolds PA, Reid CA. Effect of nonadsorbing polymers on the rheology of a concentrated nonaqueous dispersion. Langmuir. 1991; 7(1):89–94. doi:10.1021/la00049a018.
Stoliarov SI, Lyon RE, Nyden MR. A reactive molecular dynamics model of thermal decomposition in polymers. II. Polyisobutylene. Polymer. 2004;45(25):8613–8621. doi:10.1016/j.polymer.2004.10.023.
Bubálik M, Beck Á, Baladincz J, Hancsók J. Development of deposit control additives for diesel fuel. Pet Coal. 2009;51(3):167–175.
Shaikh SK, Sengstock JL. Adducts of low molecular weight PIB with low polydispersity and high vinylidene content. US Patent Application 2013/0324665 A1. 2013.
Spěváček J, Toman L, Vlěk P. 13C NMR characterization of unsaturated terminal structures in oligoisobutylenes. Polym Bull. 1995;34(4):461–467. doi:10.1007/bf00306241.
Tessier M, Maréchal E. Synthesis of mono and difunctional oligoisobutylenes—III. Modification of α-chlorooligoisobutylene by reaction with maleic anhydride. Eur Polym J. 1984;20(3):269–280. doi:10.1016/0014-3057(84)90048-X.
Tessier M, Maréchal E. Synthesis of mono and difunctional oligoisobutylenes—IV. Modification of α, ω-dichlorooligoisobutylene by reaction with maleic anhydride. Preliminary study on block polycondensation. Eur Polym J. 1984;20(3):281–290. doi:10.1016/0014-3057(84)90049-1.
Tessier M, Maréchal E. Structural study of telechelic oligoisobutylenes in relation with the experimental conditions of their synthesis. Eur Polym J. 1986;22(11):889–901. doi:10.1016/0014-3057(86)90065-0.
Harrison JJ, Mijares CM, Cheng MT, Hudson J. Negative ion electrospray ionization mass spectrum of polyisobutenylsuccinic anhydride: implications for isobutylene polymerization mechanism. Macromolecules. 2002;35(7):2494–2500. doi:10.1021/ma011799h.
Balzano F, Pucci A, Rausa R, Uccello-Barretta G. Alder-ene addition of maleic anhydride to polyisobutene: nuclear magnetic resonance evidence for an unconventional mechanism. Polym Int. 2012;61(8):1256–1262. doi:10.1002/pi.4228.
Harrison JJ, Young DC, Mayne CL. 2D-INADEQUATE structural assignment of polybutene oligomers. J Org Chem. 1997;62(3):693–699. doi:10.1021/jo961851l.
Masalova I, Kovalchuk K, Malkin AY. IR studies of interfacial interaction of the succinic surfactants with different head groups in highly concentrated W/O emulsions. J Dispers Sci Technol. 2011;32(11):1547–1555. doi:10.1080/01932691.2010.516412.
Mata JP, Reynolds PA, Gilbert EP, White JW. Extended Q-range small angle neutron scattering from inverse micellar solutions of PIBSA—Micelle and molecular scattering. Colloids Surf A. 2013;418:157–164. doi:10.1016/j.colsurfa.2012.11.034.
David C. Thermal degradation of polymers. In: Bamford CH, Tipper CFH editors. Comprehensive chemical kinetics. Amsterdam: Elsevier; 1975. p. 1–173. doi:10.1016/S0069-8040(08)70333-9.
Pirouz S, Wang Y, Chong JM, Duhamel J. Chemical modification of polyisobutylene succinimide dispersants and characterization of their associative properties. J Phys Chem B. 2015;119(37):12202–12211. doi:10.1021/acs.jpcb.5b04515.
Dimitrov P, Emert J, Hua J, Keki S, Faust R. Mechanism of isomerization in the cationic polymerization of isobutylene. Macromolecules. 2011;44(7):1831–1840. doi:10.1021/ma102645w.
Walch E, Gaymans RJ. Synthesis of low-molecular-weight telechelic polyisobutylene. Polymer. 1993;34(2):412–417. doi:10.1016/0032-3861(93)90098-U.
Warren RW, Gates DS, Driscoll GL. NMR identification of C11 to C40 branched hydrocarbons derived from the decomposition of polyisobutylene. J Polym Sci Part A-1 Polym Chem. 1971;9(3):717–746. doi:10.1002/pol.1971.150090312.
Lehrle RS, Duncan R, Liu Y, Parsons IW, Rollinson M, Lamb G, Barr D. Mass spectrometric methods for assessing the thermal stability of liquid polymers and oils: study of some liquid polyisobutylenes used in the production of crankcase oil additives. J Anal Appl Pyrol. 2002;64(2):207–227. doi:10.1016/S0165-2370(02)00032-3.
Walch E, Gaymans RJ. Telechelic polyisobutylene with unsaturated end groups and with anhydride end groups. Polymer. 1994;35(8):1774–1778. doi:10.1016/0032-3861(94)90855-9.
Chance RR, Baniukiewicz SP, Mintz D, Strate GV, Hadjichristidis N. Characterization of low-molecular-weight polymers: failure of universal calibration in size exclusion chromatography. Int J Polym Anal Charact. 1995;1(1):3–34. doi:10.1080/10236669508009704.
Acknowledgements
This work is based on the doctoral research project funded by National Research Foundation of South Africa THRIP (Grant Number 90216). We are grateful to BASF (SA) and related industries (SA) for providing samples of starting materials and industrial surfactant products. The authors wish to thank Dr. E.M. van der Merwe for assistance with the DSC studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
van der Merwe, M.M., Landman, M., van Rooyen, P.H. et al. Structural Assignment of Commercial Polyisobutylene Succinic Anhydride-based Surfactants. J Surfact Deterg 20, 193–205 (2017). https://doi.org/10.1007/s11743-016-1893-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1893-9