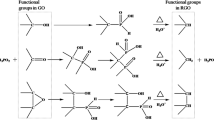
Abstract
Pencil hardness testing, electrochemical impedance spectroscopy, scanning electron microscopy, and scanning Kelvin probe microscopy were used to study the local corrosion characteristics of a graphene-oxide-modified inner coating. The effect of chloride concentration on the corrosion of the damaged inner coating was studied. The effects of chloride ions on damaged internal coatings and graphene-oxide-modified internal coatings were investigated. It was proposed to add graphene oxide into the epoxy coating to effectively inhibit the metal corrosion at the breakage. Because of the existence of graphene oxide(GO), the modified coating had a better physical property and had the effective infiltration of H2O and Cl− into the coating. The results showed that graphene oxide coatings can give X80 steel better corrosion resistance in sodium chloride solution.
摘要
采用铅笔硬度测试、电化学阻抗谱、扫描电子显微镜和扫描开尔文探针显微镜研究了氧化石墨 烯改性内涂层的局部腐蚀特性。氧化石墨烯的存在使得改性内涂层具有更高的硬度和抗划伤性能, 并 且能够有效阻隔H2O 和Cl−向涂层内部渗透, 将金属腐蚀活动限制在破损涂层处并形成一层较厚的保 护膜。结果表明, 氧化石墨烯涂层能使X80 钢在氯化钠溶液中具有较好的耐蚀性。
Similar content being viewed by others
References
ALAMRI H, IM LOW. Effect of water absorption on the mechanical properties of nano-filler reinforced epoxy nanocomposites [J]. Materials & Design, 2012, 42: 214–222. DOI: https://doi.org/10.1016/j.matdes.2012.05.060.
SHI H, LIU F, YANG L, HAN E. Characterization of protective performance of epoxy reinforced with nanometersized TiO2 and SiO2 [J]. Progress in Organic Coatings, 2008, 62(4): 359–368. DOI: https://doi.org/10.1016/j.porgcoat.2007.11.003.
SØRENSEN P A, KIIL S, DAM-JOHANSEN K, WEINELL C E. Anticorrosive coatings: A review [J]. Journal of Coatings Technology and Research, 2009, 6(2): 135–176. DOI: https://doi.org/10.1007/s11998-008-9144-2.
FENG Y, CHENG Y F. An intelligent coating doped with inhibitor-encapsulated nanocontainers for corrosion protection of pipeline steel [J]. Chemical Engineering Journal, 2017, 315: 537–551. DOI: https://doi.org/10.1016/cej.2017.01.064.
ALABBAS F M, WILLIAMSON C, BHOLA S M, SPEAR J R, OLSON D L, MISHRA B, KAKPOVBIA A E. Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80) [J]. International Biodeterioration & Biodegradation, 2013, 78: 34–42. DOI: https://doi.org/10.1016/j.ibiod.2012.10.014.
KONG D J, WU Y Z, LONG D. Stress corrosion of X80 pipeline steel welded joints by slow strain test in NACE H2S Solutions [J]. Journal of Iron and Steel Research, International, 2013, 20(1): 40–46. DOI: https://doi.org/10.1016/S1006-706X(13)60042-4.
ZHAO W, ZOU Y, MATSUDA K, ZOU Z. Corrosion behavior of reheated CGHAZ of X80 pipeline steel in H2S-containing environments [J]. Materials & Design, 2016, 99: 44–56. DOI: https://doi.org/10.1016/j.matdes.2016.03.036.
ZHAO W, ZOU Y, MATSUDA K, ZOU Z. Characterization of the effect of hydrogen sulfide on the corrosion of X80 pipeline steel in saline solution [J]. Corrosion Science, 2016, 102: 455–468. DOI: https://doi.org/10.1016/j.corsci.2015.10.038.
GUAN X, ZHANG D, WANG J, JIN Y, LI Y. Numerical and electrochemical analyses on carbon dioxide corrosion of X80 pipeline steel under different water film thicknesses in NACE solution [J]. Journal of Natural Gas Science and Engineering, 2017, 37: 199–216. DOI: https://doi.org/10.1016/j.jngse.2016.11.047.
ZAID B, SAIDI D, BENZAID A, HADJI S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy [J]. Corrosion Science, 2008, 50(7): 1841–1847. DOI: https://doi.org/10.1016/j.corsci.2008.03.006.
WANG Y F, CHENG G X, WU W, QIAO Q, LI Y, LI X F. Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions [J]. Applied Surface Science, 2015, 349: 746–756. DOI: https://doi.org/10.1016/j.apsusc.2015.05.053.
TAGHAVI N. Economic investigation on the use of internal coating for natural gas trunk-lines [J]. Chemical Engineering Research & Design, 2013, 91(9): 1725–1730. DOI: https://doi.org/10.1016/j.cherd.2013.03.012.
JI W G, HU J M, ZHANG J Q, CAO C N. Reducing the water absorption in epoxy coatings by silane monomer incorporation [J]. Corrosion Science, 2006, 48(11): 3731–3739. DOI: https://doi.org/10.1016/j.corsci.2006.02.005.
BEHZADNASAB M, MIRABEDINI S M, KABIRI K, JAMALI S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution [J]. Corrosion Science, 2011, 53(1): 89–98. DOI: https://doi.org/10.1016/j.corsci.2010.09.026.
RAMEZANZADEH B, ATTAR M M. Studying the corrosion resistance and hydrolytic degradation of an epoxy coating containing ZnO nanoparticles [J]. Materials Chemistry and Physics, 2011, 130(3): 1208–1219. DOI: https://doi.org/10.1016/j.matchemphys.2011.08.065.
ZHAO L, ZHAO L, XU Y, QIU T, ZHI L, SHI G. Polyaniline electrochromic devices with transparent graphene electrodes [J]. Electrochimica Acta, 2009, 55(2): 491–497. DOI: https://doi.org/10.1016/j.electacta.2009.08.063.
SHI X, NGUYEN T A, SUO Z, LIU Y, AVCI R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating [J]. Surface and Coatings Technology, 2009, 204(3): 237–245. DOI: https://doi.org/10.1016/j.surfcoat.2009.06.048.
YU Z, DI H, MA Y, HE Y, LIANG L, LV L, RAN X, PAN Y, LUO Z. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings [J]. Surface and Coatings Technology, 2015, 276: 471–478. DOI: https://doi.org/10.1016/j.surfcoat.2015.06.027.
RAMEZANZADEH B, AHMADI A, MAHDAVIAN M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets [J]. Corrosion Science, 2016, 109: 182–205. DOI: https://doi.org/10.1016/j.corsci.2016.04.004.
RAMEZANZADEH B, HAERI Z, RAMEZANZADEH M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance [J]. Chemical Engineering Journal, 2016, 303: 511–528. DOI: https://doi.org/10.1016/j.cej.2016.06.028.
RAMEZANZADEH B, NIROUMANDRAD S, AHMADI A, MAHDAVIAN M, MOGHADAM M H M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide [J]. Corrosion Science, 2016, 103: 283–304. DOI: https://doi.org/10.1016/j.corsci.2015.11.033.
NEMATOLLAHI M, HEIDARIAN M, PEIKARI M, KASSIRIHA S M, ARIANPOUYA N, ESMAEILPOUR M. Comparison between the effect of nanoglass flake and montmorillonite organoclay on corrosion performance of epoxy coating [J]. Corrosion Science, 2010, 52(5): 1809–1817. DOI: https://doi.org/10.1016/j.corsci.2010.01.024.
CHEN L, CHAI S, LIU K, NING N, GAO J, LIU Q, CHEN F, FU Q. Enhanced epoxy/silica composites mechanical properties by introducing graphene oxide to the interface [J]. ACS Applied Materials & Interfaces, 2012, 4(8): 4398–4404. DOI: https://doi.org/10.1021/am3010576.
MA Y, DI H, YU Z, LIANG L, LV L, PAN Y, ZHANG Y, YIN D. Fabrication of silica-decorated graphene oxide nanohybrids and the properties of composite epoxy coatings research [J]. Applied Surface Science, 2016, 360: 936–945. DOI: https://doi.org/10.1016/j.apsusc.2015.11.088.
MO M, ZHAO W, CHEN Z, YU Q, ZENG Z, WU X, XUE Q. Excellent tribological and anti-corrosion performance of polyurethane composite coatings reinforced with functionalized graphene and graphene oxide nanosheets [J]. RSC Advances, 2015, 5(70): 56486–56497. DOI: https://doi.org/10.1039/C5RA10494G.
QI K, SUN Y, DUAN H, GUO X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite [J]. Corrosion Science, 2015, 98: 500–506. DOI: https://doi.org/10.1016/j.corsci.2015.05.056.
SINGHBABU Y N, SIVAKUMAR B, SINGH J K, BAPARI H, PRAMANICK A K, SAHU R K. Efficient anti-corrosive coating of cold-rolled steel in a seawater environment using an oil-based graphene oxide ink [J]. Nanoscale, 2015, 7(17): 8035–8047. DOI: https://doi.org/10.1039/C5NR01453K.
RAJABI M, RASHED G R, ZAAREI D. Assessment of graphene oxide/epoxy nanocomposite as corrosion resistance coating on carbon steel [J]. Corrosion Engineering, Science and Technology, 2015, 50(7): 509–516. DOI: https://doi.org/10.1179/1743278214Y.0000000232.
WAN Y J, GONG L X, TANG L C, WU L B, JIANG J X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide [J]. Composites Part A: Applied Science and Manufacturing, 2014, 64: 79–89. DOI: https://doi.org/10.1016/j.compositesa.2014.04.023.
WAN Y J, TANG L C, GONG L X, YAN D, LI Y B, WU L B, JIANG J X, LAI G Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties [J]. Carbon, 2014, 69: 467–480. DOI: https://doi.org/10.1016/j.carbon.2013.12.050.
LI W, ZHOU B, WANG M, LI Z, REN R. Silane functionalization of graphene oxide and its use as a reinforcement in bismaleimide composites [J]. Journal of Materials Science, 2015, 50(16): 5402–5410. DOI: https://doi.org/10.1007/s10853-015-9084-z.
SINGH B P, JENA B K, BHATTACHARJEE S, BESRA L. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper [J]. Surface and Coatings Technology, 2013, 232: 475–481. DOI: https://doi.org/10.1016/j.surfcoat.2013.06.004.
HUANG Z Q, ZHU R G, CHEN Z, LI X Y, JING S, WANG J, HUANG X. Experimental research on the drag reduction technology of nature gas pipeline transportation [J]. Advanced Materials Research, 2012, 361-363: 982–989. DOI: https://doi.org/10.4028/www.scientific.net/AMR.361-363.982.
CHAUDHRY A U, MITTAL V, MISHRA B. Effect of graphene oxide nanoplatelets on electrochemical properties of steel substrate in saline media [J]. Materials Chemistry and Physics, 2015, 163: 130–137. DOI: https://doi.org/10.1016/j.matchemphys.2015.07.023.
TANG X, ZHOU Y, PENG M. Green preparation of epoxy/graphene oxide nanocomposites using a glycidylamine epoxy resin as the surface modifier and phase transfer agent of graphene oxide [J]. ACS Applied Materials & Interfaces, 2016, 8(3): 1854–1866. DOI: https://doi.org/10.1021/acsami.5b09830.
CHRISTOPHER G, ANBU KULANDAINATHAN M, HARICHANDRAN G. Comparative study of effect of corrosion on mild steel with waterborne polyurethane dispersion containing graphene oxide versus carbon black nanocomposites [J]. Progress in Organic Coatings, 2015, 89: 199–211. DOI: https://doi.org/10.1016/j.porgcoat.2015.09.022.
POURHASHEM S, RASHIDI A, VAEZI M R, BAGHERZADEH M R. Excellent corrosion protection performance of epoxy composite coatings filled with amino-silane functionalized graphene oxide [J]. Surface and Coatings Technology, 2017, 317: 1–9. DOI: https://doi.org/10.1016/j.surfcoat.2017.03.050.
POURHASHEM S, VAEZI M R, RASHIDI A, BAGHERZADEH M R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings [J]. Progress in Organic Coatings, 2017, 111: 47–56. DOI: https://doi.org/10.1016/j.porgcoat.2017.05.008.
GARTLEHNER G, SCHULTES M T, TITSCHER V, MORGAN L C, BOBASHEV G V, WILLIAMS P, WEST S L. User testing of an adaptation of fishbone diagrams to depict results of systematic reviews [J]. BMC Medical Research Methodology, 2017, 17(1): 169. DOI: https://doi.org/10.1186/s12874-017-0452-z.
WAN H, SONG D, LIU Z, DU C, ZENG Z, YANG X, LI X. Effect of alternating current on stress corrosion cracking behavior and mechanism of X80 pipeline steel in near-neutral solution [J]. Journal of Natural Gas Science and Engineering, 2017, 38: 458–465. DOI: https://doi.org/10.1016/j.jngse.2017.01.008.
XIE F, LI X, WANG D, WU M, SUN D. Synergistic effect of sulphate-reducing bacteria and external tensile stress on the corrosion behaviour of X80 pipeline steel in neutral soil environment [J]. Engineering Failure Analysis, 2018, 91: 382–396. DOI: https://doi.org/10.1016/j.engfailanal.2018.03.023.
ITUEN E, MKPENIE V, EKEMINI E. Corrosion inhibition of X80 steel in simulated acid wash solution using glutathione and its blends: Experimental and theoretical studies [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 578: 123597. DOI: https://doi.org/10.1016/j.colsurfa.2019.123597.
LI C, XIANG Y, SONG C, JI Z. Assessing the corrosion product scale formation characteristics of X80 steel in supercritical CO2-H2O binary systems with flue gas and NaCl impurities relevant to CCUS technology [J]. The Journal of Supercritical Fluids, 2019, 146: 107–119. DOI: https://doi.org/10.1016/j.supflu.2019.01.009.
WEI B, QIN Q, BAI Y, YU C, XU J, SUN C, KE W. Short-period corrosion of X80 pipeline steel induced by AC current in acidic red soil [J]. Engineering Failure Analysis, 2019, 105: 156–175. DOI: https://doi.org/10.1016/j.engfailanal.2019.07.014.
ZHAO S, HE L X, FAN X X, LIU C H, LONG J P, WANG L, CHANG H, WANG J, ZHANG W. Microstructure and chloride corrosion property of nanocrystalline AlTiCrNiTa high entropy alloy coating on X80 pipeline steel [J]. Surface and Coatings Technology, 2019, 375: 215–220. DOI: https://doi.org/10.1016/j.surfcoat.2019.07.033.
Author information
Authors and Affiliations
Contributions
LIAO Ke-xi provided the concept and edited the draft of manuscript. LI Xiao-xiao completed the experiment of the paper. JIANG Yi provided directional guidance and financial support for the whole study. LIU Xin, JIANG Yi and JING Hong edited the pictures. LIAO Ke-xi examined the manuscript, and LI Xiao-xiao provided directional guidance.
Corresponding author
Additional information
Conflict of interest
The authors declare no conflicts of interest.
Foundation item
Project(51674212) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Liao, Kx., Li, Xx., Jiang, Y. et al. Local corrosion characteristics of a graphene-oxide-modified inner coating. J. Cent. South Univ. 27, 3213–3226 (2020). https://doi.org/10.1007/s11771-020-4541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-020-4541-5