Abstract
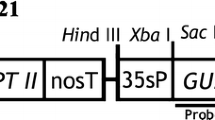
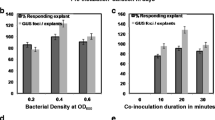
Genetic transformation is a tool of special interest for developing new biotechnological strategies for the production of bio-active compounds such as cardenolides, which are exclusively obtained from plants. To date, Digitalis plants are the main economically viable source of cardenolides for the pharmaceutical industry. This study describes the development of efficient plant regeneration and Agrobacterium-mediated genetic transformation protocols for Digitalis purpurea L. First, a plant regeneration procedure starting from leaf segments of in vitro-cultivated plants was established and the minimal inhibitory concentration of G-418 (geneticin) for callus induction was determined. Both leaf segments and callus tissue were sensitive to G-418 70 mg l−1. Afterwards, two Agrobacterium strains were used to test their T-DNA transfer ability on D. purpurea leaf tissues, EHA105 and C58C1RifR (pMP90), both harboring the binary vector pTJK136. Strain C58C1RifR (pMP90) yielded a higher number of transformed plants than EHA105. Successful transformation was confirmed by histochemical β-glucuronidase (GUS) assays of the putative transgenic tissues and PCR analyses using β-glucuronidase (uidA)- and neomycin phosphotransferase II (nptII)-specific primers. Southern blot hybridization confirmed the stable integration of the nptII gene in the transgenic plants. In total, 518 independent transgenic lines were regenerated with an average of 6.91 transgenic lines per initial leaf segment infected with A. tumefaciens strain C58C1RifR (pMP90). To date, only a few studies have been published on the genetic transformation of Digitalis species. The protocols for plant regeneration and genetic transformation described in this paper will contribute to functional studies for a better understanding of cardenolide biosynthetic pathways and the metabolic engineering of cardenolides to develop high-yielding improved genotypes.




Similar content being viewed by others
References
Abou-Alaiwi WA, Potlakayala SD, Goldman SL, Josekutty PC, Karelia DN, Rudrabhatla SV (2012) Agrobacterium-mediated transformation of the medicinal plant Centaurea montana. Plant Cell Tissue Organ Cult 109:1–8
Andrade GM, Nairn CJ, Le HT, Merkle SA (2009) Sexually mature transgenic American chestnut trees via embryogenic suspension-based transformation. Plant Cell Rep 28:1385–1397
Boszoradova E, Libantova J, Matusikova I, Poloniova Z, Jopcik M, Berenyi M, Moravcikova J (2011) Agrobacterium-mediated genetic transformation of economically important oilseed rape cultivars. Plant Cell Tissue Organ Cult 107:317–323
Canter PH, Thomas H, Ernst E (2005) Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol 23:180–185
Chetty VJ, Ceballos N, García D, Narváez-Vásquez J, López W, Orozco-Cárdenas ML (2012) Evaluation of four Agrobacterium tumefaciens strains for the genetic transformation of tomato (Solanum lycopersicum L.) cultivar Micro-Tom. Plant Cell Rep 34:747–754
Chong-Pérez B, Reyes M, Rojas L, Ocaña B, Pérez B, Kosky RG, Angenon G (2012) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in banana cv. ‘‘Dwarf Cavendish’’ (Musa Cavendish, AAA): effect of spermidine on transformation efficiency. Plant Cell Tissue Organ Cult 111:79–90
De Bondt A, Eggermont K, Druart P, De Vil M, Goderis I, Vanderleyden J, Broekaert WF (1994) Agrobacterium-mediated transformation of apple (Malus × domestica Borkh.): an assessment of factors affecting gene transfer efficiency during early transformation steps. Plant Cell Rep 13:587–593
Elliott AR, Campbell JA, Brettell RIS, Grof CPL (1998) Agrobacterium-mediated transformation of sugarcane using GFP as a screenable marker. Aust J Plant Physiol 25:739–743
Fahim M, Ayala-Navarrete L, Millar AA, Larkin PJ (2010) Hairpin RNA derived from viral NIa gene confers immunity to wheat streak mosaic virus infection in transgenic wheat plants. Plant Biotechnol J 8:821–834
Fatima Z, Mujib A, Fatima S, Arshi A, Umar S (2009) Callus induction, biomass growth, and plant regeneration in Digitalis lanata Ehrh.: influence of plant growth regulators and carbohydrates. Turk J Bot 33:393–405
Gatica-Arias A, Farag MA, Stanke M, Matoušek J, Wessjohann L, Weber G (2012) Flavonoid production in transgenic hop (Humulus lupulus L.) altered by PAP1/MYB75 from Arabidopsis thaliana L. Plant Cell Rep 31:111–119
Gavidia I, Tarrio R, Rodríguez-Trelles F, Pérez-Bermúdez P, Seitz HU (2007) Plant progesterone 5β-reductase is not homologous to the animal enzyme. Molecular evolutionary characterization of P5βR from Digitalis purpurea. Phytochemistry 68:853–864
Gelvin SB (2003) Improving plant genetic engineering by manipulating the host. Trends Biotechnol 21:95–98
Gelvin SB, Kim S-I (2007) Effect of chromatin upon Agrobacterium T-DNA integration and transgene expression. Biochim Biophys Acta 1769:410–421
Gómez-Galera S, Pelacho AM, Gene A, Capell T, Christou P (2007) The genetic manipulation of medicinal and aromatic plants. Plant Cell Rep 26:1689–1715
Gurel E, Yücesan B, Aglic E, Gurel S, Verma SK, Sokmen M, Sokmen A (2011) Regeneration and cardiotonic glycoside production in Digitalis davisiana Heywood (Alanya Foxglove). Plant Cell Tissue Organ Cult 104:217–225
Hagimori M, Matsumoto T, Obi Y (1983) Effects of mineral salts, initial pH and precursors on digitoxin formation by shoot-forming cultures of Digitalis purpurea L. grown in liquid media. Agricult Biol Chem 47:565–571
Han JL, Liu BY, Ye HC, Wang H, Li ZQ, Li GF (2006) Effects of overexpression of the endogenous farnesyl diphosphate synthase on the artemisin content in Artemisia annua L. J Integr Plant Biol 48:482–487
Herl V, Frankenstein J, Meitinger N, Muller-Uri F, Kreis W (2007) Δ5-3β-Hydroxysteroid dehydrogenase (3βHSD) from Digitalis lanata. Heterologous expression and characterization of the recombinant enzyme. Planta Med 73:704–710
Herrera MT, Cacho M, Corchete MP, Fernández-Tarrago J (1990) One step shoot tip multiplication and rooting of Digitalis thapsi L. Plant Cell Tissue Org Cult 22:179–182
Hood EE, Gelvin SB, Melehers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jiménez V (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Kapila J, De Rycke R, Van Montagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122:101–108
Khayat E, Duvdevani A, Lehav E, Ballesteros BA (2004) Somaclonal variation in banana (Musa acuminata cv. Grande Naine). Genetic mechanism, frequency, and application as a tool for clonal selection. In: Jain SM, Swennen R (eds) Banana improvement: cellular, molecular biology, and induced mutation. Science, Plymouth, pp 99–109
Koga M, Hirashima K, Nakahara T (2000) The transformation system in Foxglove (Digitalis purpurea L.) using Agrobacterium rhizogenes and traits of the regenerants. Plant Biotechnol 17:99–104
Komari T, Ishida Y, Hiei Y (2004) Plant transformation technology: Agrobacterium-mediated transformation. In: Christou P, Klee H (eds) Handbook of plant biotechnology 1. Wiley, London, pp 233–261
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Kreis W, Müller-Uri F (2013) Cardenolide aglycone formation in Digitalis. In: Bach TJ, Rohmer M (eds) Isoprenoid synthesis in plants and microorganisms: new concepts and experimental approaches. Springer, New York, pp 425–438
Kuate SP, Padua RM, Eisenbeiss WF, Kreis W (2008) Purification and characterization of malonyl-coenzyme A: 21-hydroxypregnane 21-O-malonyltransferase (Dp21MaT) from leaves of Digitalis purpurea L. Phytochemistry 69:619–626
Lee L-Y, Gelvin SB (2008) T-DNA binary vectors and systems. Plant Physiol 146:325–332
Lehmann U, Moldenhauer D, Thomar S, Diettrich B, Luckner M (1995) Regeneration of plants from Digitalis lanata cells transformed with Agrobacterium tumefaciens carrying bacterial genes encoding neomycin phosphotransferase II and β-glucuronidase. J Plant Physiol 147:53–57
Li WJ, Dai LL, Chai ZJ, Yin ZJ, Qu LQ (2012) Evaluation of seed storage protein gene 3´-untranslated regions in enhancing gene expression in transgenic rice seed. Transgenic Res 21:545–553
Li Y, Gao Z, Piao C, Lu K, Wang Z, Cui M-L (2014) A stable and efficient Agrobacterium tumefaciens-mediated genetic transformation of the medicinal plant Digitalis purpurea L. Appl Biochem Biotechnol 172:1807–1817
Linsmaier E, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Liu X, Brost J, Hutcheon C, Guilfoil R, Wilson AK, Leung S, Shewmaker CK, Rooke S, Nguyen T, Kiser J, De Rocher J (2012) Transformation of the oilseed crop Camelina sativa by Agrobacterium-mediated floral dip and simple large-scale screening of transformants. In Vitro Cell Dev Biol-Plant 48:462–468
Maheshwari P, Selvaraj G, Kovalchuk I (2011) Optimization of Brassica napus (canola) segment regeneration for genetic transformation. New Biotechnol 29:144–155
Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, Schlemmer F, Sulpice E, Locher C, Gidrol X, Ghiringhelli F, Modjtahedi N, Galluzzi L, André F, Zitvogel L, Kepp O, Kroemer G (2012) Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med 4:143ra99. doi:10.1126/scitranslmed.3003807
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nadolska-Orczyk A, Orczyk W (2000) Study of the factors influencing Agrobacterium-mediated transformation of pea (Pisum sativum L.). Mol Breed 6:185–194
Nandy S, Srivastava V (2012) Marker-free site-specific gene integration in rice based on the use of two recombination systems. Plant Biotechnol J 10:904–912
Padilla IMG, Burgos L (2010) Aminoglycoside antibiotics: structure, functions and effects on in vitro plant culture and genetic transformation protocols. Plant Cell Rep 29:1203–1213
Patil JG, Ahire ML, Nitnaware KM, Panda S, Bhatt VP, Kavi Kishor PB, Nikam TD (2013) In vitro propagation and production of cardiotonic glycosides in shoot cultures of Digitalis purpurea L. by elicitation and precursor feeding. Appl Microbiol Biotechnol 97:2379–2393
Pérez-Alonso N, Wilken D, Gerth A, Jahn A, Nitzsche HM, Kerns G, Capote A, Jiménez E (2009) Cardiotonic glycosides from biomass of Digitalis purpurea L. cultured in temporary immersion systems. Plant Cell Tissue Org Cult 99:151–156
Pérez-Bermúdez P, Moya García A, Tuñón I, Gavidia I (2010) Digitalis purpurea P5BR2, encoding steroid 5β-reductase, is a novel defense-related gene involved in cardenolide biosynthesis. New Phytol 185:687–700
Pradel H, Lehmann U, Diettrich B, Luckner M (1997) Hairy root cultures of Digitalis lanata: secondary metabolism and plant regeneration. J Plant Physiol 151:209–215
Prassas I, Diamandis EP (2008) Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov 7:926–935
Rajesh M, Jeyaraj M, Sivanandhan G, Subramanyam K, Mariashibu TS, Mayavan S, Kapil Dev G, Anbazhagan VR, Manickavasagam M, Ganapathi A (2013) Agrobacterium-mediated transformation of the medicinal plant Podophyllum hexandrum Royle (syn. P. emodi Wall. ex Hook.f. & Thomas). Plant Cell Tissue Organ Cult 114:71–82
Roca-Pérez L, Boluda R, Gavidia I, Pérez-Bermúdez P (2004) Seasonal cardenolide production and Dop5βr gene expression in natural populations of Digitalis obscura. Phytochemistry 65:1869–1878
Rosellini D (2012) Selectable markers and reporter genes: a well furnished toolbox for plant science and genetic engineering. Crit Rev Plant Sci 31:401–453
Saito K, Shimomura MYK, Yoshimatsu K, Murakoshi I (1990) Genetic transformation of foxglove (Digitalis purpurea) by chimeric foreign genes and production of cardioactive glycosides. Plant Cell Rep 9:121–124
Sales E, Segura J, Arillaga I (2003) Agrobacterium-mediated genetic transformation of the cardenolide-producing plant Digitalis minor. Planta Med 69:143–147
Sales E, Muñoz-Bertomeu J, Arrillaga I, Segura J (2007) Enhancement of cardenolide and phytosterol levels by expression of an N-terminally truncated 3-hydroxy-3-methyglutaryl CoA reductase in transgenic Digitalis minor. Planta Med 73:605–610
Sales E, Müller-Uri F, Nebauer SG, Segura J, Kreis W, Arillaga I (2011) Digitalis. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, plantation and ornamental crops. Springer, Berlin, pp 73–112
Song G, Walworth A (2013) Agrobacterium tumefaciens-mediated transformation of Atropa belladonna. Plant Cell Tissue Organ Cult 115:107–113
Tadesse Y, Sagi L, Swennen R, Jacobs M (2003) Optimisation of transformation conditions and production of transgenic sorghum (Sorghum bicolor) via microparticle bombardment. Plant Cell Tissue Organ Cult 75:1–18
Van der Frits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Vancanneyt G, Schmidt R, O’Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220:245–250
Verma SK, Yücesan B, Gurel S, Gurel E (2011) Indirect somatic embryogenesis and shoot organogenesis from cotyledonary leaf segments of Digitalis lamarckii Ivan., an endemic medicinal species. Turk J Biol 35:743–750
Warren B (2005) Digitalis purpurea. Am J Cardiol 95:544
Wu B, Li Y, Yan H, Ma Y, Luo H, Yuan L, Chen S, Lu S (2012) Comprehensive transcriptome analysis reveals novel genes involved in cardiac glycoside biosynthesis and mlncRNAs associated with secondary metabolism and stress response in Digitalis purpurea. BMC Genom 13:15
Xue GP, Way HM, Richardson T, Joyce PA, Drenth J, McIntyre CL (2011) Overexpression of TaNAC69 leads to enhanced transcript levels of stress up-regulated genes and dehydration tolerance in bread wheat. Mol Plant 4:697–712
Acknowledgments
The authors thank the support of the EU through the ALFA Network CARIBIOTEC (project AML/B7-311/97/0666/II-0201) and the Institutional University Collaboration programme with Universidad Central “Marta Abreu” de Las Villas funded by the Flemish Interuniversity Council (VLIR-IUC UCLV).
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Pérez-Alonso and B. Chong-Pérez contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Pérez-Alonso, N., Chong-Pérez, B., Capote, A. et al. Agrobacterium tumefaciens-mediated genetic transformation of Digitalis purpurea L.. Plant Biotechnol Rep 8, 387–397 (2014). https://doi.org/10.1007/s11816-014-0329-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-014-0329-0