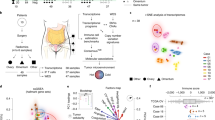
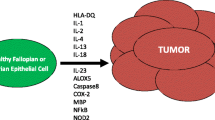
Opinion statement
Ovarian cancer (OC), especially high-grade serous cancer (HGSC), is a highly heterogeneous malignancy with limited options for curative treatment and a high frequency of relapse. Interactions between OC and the immune system may permit immunoediting and immune escape, and current standard of care therapies can influence immune cell infiltration and function within the tumor microenvironment. Natural killer (NK) cells are involved in cancer immunosurveillance and immunoediting and can be activated by therapy, but deliberate approaches to maximize NK cell reactivity for treatment of HGSC are in their infancy. NK cells may be the ideal target for immunotherapy of HGSC. The diverse functions of NK cells, and their established roles in immunosurveillance, make them attractive candidates for more precise and effective HGSC treatment. NK cells’ functional capabilities differ because of variation in receptor expression and genetics, with meaningful impacts on their anticancer activity. Studying HGSC:NK cell interactions will define the features that predict the best outcomes for patients with the disease, but the highly diverse nature of HGSC will likely require combination therapies or approaches to simultaneously target multiple, co-existing features of the tumor to avoid tumor escape and relapse. We expect that the ideal therapy will enable NK cell infiltration and activity, reverse immunosuppression within the tumor microenvironment, and enable effector functions against the diverse subpopulations that comprise HGSC.

Similar content being viewed by others
References and Recommended Reading
West K, Borley J. Ovarian, fallopian tube and primary peritoneal cancer: an overview. Obstetrics, Gynaecology & Reproductive Medicine. 2020;30(12):380–6. https://doi.org/10.1016/j.ogrm.2020.10.001.
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–96. https://doi.org/10.3322/caac.21456.
Koshiyama M, Matsumura N, Konishi I. Recent concepts of ovarian carcinogenesis: Type i and type ii. BioMed Research International. 2014;2014:934261. https://doi.org/10.1155/2014/934261.
Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460(3):237–49. https://doi.org/10.1007/s00428-012-1203-5.
Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nature Reviews Cancer. 2009;9(6):415–28. https://doi.org/10.1038/nrc2644.
van der Burg MEL, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, Lacave AJ, Nardi M, Renard J, Pecorelli S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. New England Journal of Medicine. 1995;332(10):629–34. https://doi.org/10.1056/nejm199503093321002.
Lee EK, Matulonis UA. Emerging drugs for the treatment of ovarian cancer: a focused review of parp inhibitors. Expert Opin Emerg Drugs. 2020;25(2):165–88. https://doi.org/10.1080/4728214.2020.1773791.
Fuh KC, Secord AA, Bevis KS, Huh W, ElNaggar A, Blansit K, Previs R, Tillmanns T, Kapp DS, Chan JK. Comparison of bevacizumab alone or with chemotherapy in recurrent ovarian cancer patients. Gynecol Oncol. 2015;139(3):413–8. https://doi.org/10.1016/j.ygyno.2015.06.041.
Mirza MR, Coleman RL, Gonzalez-Martin A, Moore KN, Colombo N, Ray-Coquard I, Pignata S. The forefront of ovarian cancer therapy: update on parp inhibitors. Ann Oncol. 2020;31(9):1148–59. https://doi.org/10.1016/j.annonc.2020.06.004.
Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS, Birrer M, Provencher DM, Sehouli J, Colombo N, Gonzalez-Martin A, Oaknin A, Ottevanger PB, Rudaitis V, Katchar K, Wu H, Keefe S, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase ii keynote-100 study. Ann Oncol. 2019;30(7):1080–7. https://doi.org/10.1093/annonc/mdz135.
Clouthier DL, Lien SC, Yang SYC, Nguyen LT, Manem VSK, Gray D, Ryczko M, Razak ARA, Lewin J, Lheureux S, Colombo I, Bedard PL, Cescon D, Spreafico A, Butler MO, Hansen AR, Jang RW, Ghai S, Weinreb I, et al. An interim report on the investigator-initiated phase 2 study of pembrolizumab immunological response evaluation (inspire). J Immunother Cancer. 2019;7(1):72. https://doi.org/10.1186/s40425-019-0541-0.
Liu J, Wang Y, Yuan S, Wei J, Bai J. Construction of an immune cell infiltration score to evaluate the prognosis and therapeutic efficacy of ovarian cancer patients. Front Immunol. 2021;12:751594. https://doi.org/10.3389/fimmu.2021.751594.
Nersesian S, Lee SN, Grantham SR, Meunier L, Communal L, Arnason T, Nelson BH, Mes-Masson AM, Boudreau JE, Boudreau JE. Cd16ahigh nk cell infiltration and spatial relationships with t cells and macrophages can predict improved progression-free survival in high grade ovarian cancer. MedRxiv. 2021. https://doi.org/10.1101/2021.06.08.21258566.
Laumont CM, Wouters MCA, Smazynski J, Gierc NS, Chavez EA, Chong LC, Thornton S, Milne K, Webb JR, Steidl C, Nelson BH. Single-cell profiles and prognostic impact of tumor-infiltrating lymphocytes coexpressing cd39, cd103, and pd-1 in ovarian cancer. Clin Cancer Res. 2021;27(14):4089–100. https://doi.org/10.1158/1078-0432.CCR-20-4394.
Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic t-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. 2016;22(12):3005–15. https://doi.org/10.1158/1078-0432.CCR-15-2762.
Gaudreau PO, Allard B, Turcotte M, Stagg J. Cd73-adenosine reduces immune responses and survival in ovarian cancer patients. Oncoimmunology. 2016;5(5):e1127496. https://doi.org/10.1080/2162402X.2015.1127496.
Crome SQ, Nguyen LT, Lopez-Verges S, Yang SY, Martin B, Yam JY, Johnson DJ, Nie J, Pniak M, Yen PH, Milea A, Sowamber R, Katz SR, Bernardini MQ, Clarke BA, Shaw PA, Lang PA, Berman HK, Pugh TJ, et al. A distinct innate lymphoid cell population regulates tumor-associated t cells. Nat Med. 2017;23(3):368–75. https://doi.org/10.1038/nm.4278.
Banville AC, Wouters MCA, Oberg AL, Goergen KM, Maurer MJ, Milne K, Ashkani J, Field E, Ghesquiere C, Jones SJM, Block MS, Nelson BH. Co-expression patterns of chimeric antigen receptor (car)-t cell target antigens in primary and recurrent ovarian cancer. Gynecol Oncol. 2021;160(2):520–9. https://doi.org/10.1016/j.ygyno.2020.12.005.
Zhang AW, McPherson A, Milne K, Kroeger DR, Hamilton PT, Miranda A, Funnell T, Little N, de Souza CPE, Laan S, LeDoux S, Cochrane DR, Lim JLP, Yang W, Roth A, Smith MA, Ho J, Tse K, Zeng T, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell. 2018;173(7):1755–1769 e22. https://doi.org/10.1016/j.cell.2018.03.073.
Nath A, Cosgrove PA, Mirsafian H, Christie EL, Pflieger L, Copeland B, Majumdar S, Cristea MC, Han ES, Lee SJ, Wang EW, Fereday S, Traficante N, Salgia R, Werner T, Cohen AL, Moos P, Chang JT, Bowtell DDL, Bild AH. Evolution of core archetypal phenotypes in progressive high grade serous ovarian cancer. Nat Commun. 2021;12(1):3039. https://doi.org/10.1038/s41467-021-23171-3.
Sarivalasis A, Morotti M, Mulvey A, Imbimbo M, Coukos G. Cell therapies in ovarian cancer. Ther Adv Med Oncol. 2021;13:17588359211008399. https://doi.org/10.1177/17588359211008399.
Testa U, Petrucci E, Pasquini L, Castelli G, Pelosi E. Ovarian cancers: genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines (Basel). 2018;5(1). https://doi.org/10.3390/medicines5010016.
Schuijer M, Berns EMJJ. Tp53 and ovarian cancer. Human Mutation. 2003;21(3):285–91. https://doi.org/10.1002/humu.10181.
Alexandrov, LB, Nik-Zainal, S, Wedge, DC, Aparicio, SAJR, Behjati, S, Biankin, AV, Bignell, GR, Bolli, N, Borg, A, Børresen-Dale, A-L, Boyault, S, Burkhardt, B, Butler, AP, Caldas, C, Davies, HR, Desmedt, C, Eils, R, Eyfjörd, JE, Foekens, JA, Greaves, M, Hosoda, F, Hutter, B, Ilicic, T, Imbeaud, S, Imielinski, M, Jäger, N, Jones, DTW, Jones, D, Knappskog, S, Kool, M, Lakhani, SR, López-Otín, C, Martin, S, Munshi, NC, Nakamura, H, Northcott, PA, Pajic, M, Papaemmanuil, E, Paradiso, A, Pearson, JV, Puente, XS, Raine, K, Ramakrishna, M, Richardson, AL, Richter, J, Rosenstiel, P, Schlesner, M, Schumacher, TN, Span, PN, Teague, JW, Totoki, Y, Tutt, ANJ, Valdés-Mas, R, van Buuren, MM, van ’t Veer, L, Vincent-Salomon, A, Waddell, N, Yates, LR, Zucman-Rossi, J, Andrew Futreal, P, McDermott, U, Lichter, P, Meyerson, M, Grimmond, SM, Siebert, R, Campo, E, Shibata, T, Pfister, SM, Campbell, PJ, Stratton, MR, Australian Pancreatic Cancer Genome, I, Consortium, IBC, Consortium, IM-S, PedBrain, I, Signatures of mutational processes in human cancer, Nature 500(7463) (2013) 415-421. https://doi.org/10.1038/nature12477
Cancer Genome Atlas Research. N, Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. https://doi.org/10.1038/nature10166.
Nath A, Cosgrove PA, Mirsafian H, Christie EL, Pflieger L, Copeland B, Majumdar S, Cristea MC, Han ES, Lee SJ, Wang EW, Fereday S, Traficante N, Salgia R, Werner T, Cohen AL, Moos P, Chang JT, Bowtell DDL, Bild AH. Evolution of core archetypal phenotypes in progressive high grade serous ovarian cancer. Nature Communications. 2021;12(1):3039. https://doi.org/10.1038/s41467-021-23171-3.
Lee S, Zhao L, Rojas C, Bateman NW, Yao H, Lara OD, Celestino J, Morgan MB, Nguyen TV, Conrads KA, Rangel KM, Dood RL, Hajek RA, Fawcett GL, Chu RA, Wilson K, Loffredo JL, Viollet C, Jazaeri AA, et al. Molecular analysis of clinically defined subsets of high-grade serous ovarian cancer. Cell Rep. 2020;31(2):107502. https://doi.org/10.1016/j.celrep.2020.03.066.
Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, Fereday S, Lawrence M, Carter SL, Mermel CH, Kostic AD, Etemadmoghadam D, Saksena G, Cibulskis K, Duraisamy S, Levanon K, Sougnez C, Tsherniak A, Gomez S, et al. Cancer Genome Atlas Research, N, Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123(1):517–25. https://doi.org/10.1172/JCI65833.
Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nature Communications. 2013;4(1):2126. https://doi.org/10.1038/ncomms3126.
Zhou X, Qu M, Tebon P, Jiang X, Wang C, Xue Y, Zhu J, Zhang S, Oklu R, Sengupta S, Sun W, Khademhosseini A. Screening cancer immunotherapy: When engineering approaches meet artificial intelligence. Adv Sci (Weinh). 2020;7(19):2001447. https://doi.org/10.1002/advs.202001447.
Rodenhizer D, Dean T, Xu B, Cojocari D, McGuigan AP. A three-dimensional engineered heterogeneous tumor model for assessing cellular environment and response. Nat Protoc. 2018;13(9):1917–57. https://doi.org/10.1038/s41596-018-0022-9.
Ayuso JM, Rehman S, Virumbrales-Munoz M, McMinn PH, Geiger P, Fitzgerald C, Heaster T, Skala MC, Beebe DJ. Microfluidic tumor-on-a-chip model to evaluate the role of tumor environmental stress on nk cell exhaustion, Science. Advances. 2021;17(7):eabc2331. https://doi.org/10.1126/sciadv.abc2331.
Wang Y, Jin R, Shen B, Li N, Zhou H, Wang W, Zhao Y, Huang M, Fang P, Wang S, Mary P, Wang R, Ma P, Li R, Tian Y, Cao Y, Li F, Schwieizer L, Zhang H. High-throughput functional screening for next-generation cancer immunotherapy using droplet-based microfluidics. Science Advances 7. 2021:eabe3839. https://doi.org/10.1126/sciadv.abe3839.
Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of h-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–8. https://doi.org/10.1038/319675a0.
Kannan GS, Aquino-Lopez A, Lee DA. Natural killer cells in malignant hematology: a primer for the non-immunologist. Blood Reviews. 2017;31(2):1–10. https://doi.org/10.1016/j.blre.2016.08.007.
Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, Kim S. Epigenetic modification and antibody-dependent expansion of memory-like nk cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–42. https://doi.org/10.1016/j.immuni.2015.02.013.
Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath T, Simpson L, Haigwood NL, Blish CA, Akbar AN, Paust S. Human natural killer cells mediate adaptive immunity to viral antigens. Sci Immunol. 2019;4(35). https://doi.org/10.1126/sciimmunol.aat8116.
Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath T, Simpson L, Haigwood NL, Blish CA, Akbar AN, Paust S. Human natural killer cells mediate adaptive immunity to viral antigens. Science Immunology. 2019;4(35):eaat8116. https://doi.org/10.1126/sciimmunol.aat8116.
Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee Y-S, Mulder A, Claas F, Cooper MA, Fehniger TA. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Science Translational Medicine. 2016;8(357):357ra123-357ra123. https://doi.org/10.1126/scitranslmed.aaf2341.
Marin ND, Krasnick BA, Becker-Hapak M, Conant L, Goedegebuure SP, Berrien-Elliott MM, Robbins KJ, Foltz JA, Foster M, Wong P, Cubitt CC, Tran J, Wetzel CB, Jacobs M, Zhou AY, Russler-Germain D, Marsala L, Schappe T, Fields RC, Fehniger TA. Memory-like differentiation enhances nk cell responses to melanoma. Clin Cancer Res. 2021. https://doi.org/10.1158/1078-0432.CCR-21-0851.
Boudreau JE, Hsu KC. Natural killer cell education and the response to infection and cancer therapy: stay tuned. Trends in Immunology. 2018;39(3):222–39. https://doi.org/10.1016/j.it.2017.12.001.
Gasser S. DNA damage response and development of targeted cancer treatments. Annals of Medicine. 2007;39(6):457–64. https://doi.org/10.1080/07853890701436773.
Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, Zhang L, Iannello A, Mathur N, Jardine KE, Kirn GA, Bell JC, McBurney MW, Raulet DH, Ardolino M. Contribution of nk cells to immunotherapy mediated by pd-1/pd-l1 blockade. J Clin Invest. 2018;128(10):4654–68. https://doi.org/10.1172/JCI99317.
Sanchez-Correa B, Morgado S, Gayoso I, Bergua JM, Casado JG, Arcos MJ, Bengochea ML, Duran E, Solana R, Tarazona R. Human nk cells in acute myeloid leukaemia patients: Analysis of nk cell-activating receptors and their ligands. Cancer Immunol Immunother. 2011;60(8):1195–205. https://doi.org/10.1007/s00262-011-1050-2.
Sanchez-Correa B, Valhondo I, Hassouneh F, Lopez-Sejas N, Pera A, Bergua JM, Arcos MJ, Banas H, Casas-Aviles I, Duran E, Alonso C, Solana R, Tarazona R. Dnam-1 and the tigit/pvrig/tactile axis: novel immune checkpoints for natural killer cell-based cancer immunotherapy. Cancers (Basel). 2019;11(6). https://doi.org/10.3390/cancers11060877.
Boudreau JE, Hsu KC. Natural killer cell education in human health and disease. Current Opinion in Immunology. 2018;50:102–11. https://doi.org/10.1016/j.coi.2017.11.003.
Crome S, Nguyen L, López-Vergès S, Yang SYC, Martin B, Yam J, Johnson D, Nie J, Pniak M, Yen P, Milea A, Sowamber R, Katz S, Bernardini M, Clarke B, Shaw P, Lang P, Berman H, Pugh T, Ohashi P. A distinct innate lymphoid cell population regulates tumor-associated t cells. Nature Medicine. 2017;23. https://doi.org/10.1038/nm.4278.
Nersesian S, Schwartz SL, Grantham SR, MacLean LK, Lee SN, Pugh-Toole M, Boudreau JE. Nk cell infiltration is associated with improved overall survival in solid cancers: a systematic review and meta-analysis. Transl Oncol. 2021;14(1):100930. https://doi.org/10.1016/j.tranon.2020.100930.
Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, Davis MM, Norman PJ, Guethlein LA, Desai M, Parham P, Blish CA. Genetic and environmental determinants of human nk cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5(208):208ra145. https://doi.org/10.1126/scitranslmed.3006702.
Smith SL, Philippa RK, Stacey KB, Worboys JD, Yarwood A, Seo S, Hegewisch Solloa E, Mistretta B, Chatterjee SS, Gunaratne P, Allette K, Wang Y-C, Laird Smith M, Sebra R, Mace EM, Horowitz A, Thomson W, Martin P, Eyre S, Davis DM. Diversity of periphal blood human nk cells identified by single-cell rna sequencing. Blood Adv. 2020;4(7):1388–406. https://doi.org/10.1182/bloodadvances.2019000699.
Wagner JA, Berrien-Elliott MM, Rosario M, Leong JW, Jewell BA, Schappe T, Abdel-Latif S, Fehniger TA. Cytokine-induced memory-like differentiation enhances unlicensed natural killer cell antileukemia and fcgammariiia-triggered responses. Biol Blood Marrow Transplant. 2017;23(3):398–404. https://doi.org/10.1016/j.bbmt.2016.11.018.
Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human ipsc-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192.e5. https://doi.org/10.1016/j.stem.2018.06.002.
Miller JS, Bjordahl R, Gaidarova S, Mahmood S, Rogers P, Moyar G, Blazar BR, Kaufman DS, Valamehr B, Cichocki F. Ipsc-derived nk cells synergize with t cells and anti-pd-1 antibody to mediate durable anti-tumor responses in vivo. Blood. 2019;134(Supplement_1):1933–3. https://doi.org/10.1182/blood-2019-124961.
Shah N, Li L, McCarty J, Kaur I, Yvon E, Shaim H, Muftuoglu M, Liu E, Orlowski RZ, Cooper L, Lee D, Parmar S, Cao K, Sobieiski C, Saliba R, Hosing C, Ahmed S, Nieto Y, Bashir Q, et al. Phase i study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177(3):457–66. https://doi.org/10.1111/bjh.14570.
Gasser S, Raulet DH. The DNA damage response arouses the immune system. Cancer Research. 2006;66(8):3959–62. https://doi.org/10.1158/0008-5472.Can-05-4603.
Xing S. Ferrari de Andrade, L, Nkg2d and mica/b shedding: a ‘tag game’ between nk cells and malignant cells. Clin Transl Immunology. 2020;9(12):e1230. https://doi.org/10.1002/cti2.1230.
Le Luduec J-B, Boudreau JE, Freiberg JC, Hsu KC. Novel approach to cell surface discrimination between kir2dl1 subtypes and kir2ds1 identifies hierarchies in nk repertoire, education, and tolerance. Frontiers in Immunology. 2019;10(734). https://doi.org/10.3389/fimmu.2019.00734.
Shaffer BC, Le Luduec JB, Park S, Devlin S, Archer A, Davis E, Cooper C, Nhaissi M, Suri B, Wells D, Tamari R, Papadopoulos E, Jakubowski AA, Giralt S, Hsu KC. Prospective kir genotype evaluation of hematopoietic cell donors is feasible with potential to benefit patients with aml. Blood Adv. 2021;5(7):2003–11. https://doi.org/10.1182/bloodadvances.2020002701.
Morales-Estevez C, De la Haba-Rodriguez J, Manzanares-Martin B, Porras-Quintela I, Rodriguez-Ariza A, Moreno-Vega A, Ortiz-Morales MJ, Gomez-Espana MA, Cano-Osuna MT, Lopez-Gonzalez J, Chia-Delgado B, Gonzalez-Fernandez R, Aranda-Aguilar E. Kir genes and their ligands predict the response to anti-egfr monoclonal antibodies in solid tumors. Front Immunol. 2016;7:561. https://doi.org/10.3389/fimmu.2016.00561.
Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, Modak S, Heller G, Dupont B, Cheung NK, Hsu KC. Unlicensed nk cells target neuroblastoma following anti-gd2 antibody treatment. J Clin Invest. 2012;122(9):3260–70. https://doi.org/10.1172/JCI62749.
Pesce S, Tabellini G, Cantoni C, Patrizi O, Coltrini D, Rampinelli F, Matta J, Vivier E, Moretta A, Parolini S, Marcenaro E. B7-h6-mediated downregulation of nkp30 in nk cells contributes to ovarian carcinoma immune escape. OncoImmunology. 2015;4(4):e1001224. https://doi.org/10.1080/2162402X.2014.1001224.
Wang Y-J, Fletcher R, Yu J, Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes & Diseases. 2018;5(3):194–203. https://doi.org/10.1016/j.gendis.2018.05.003.
Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, Els NV, Phillips MD, Schweizer C, Weil SC, Larson SM, Old LJ. Farletuzumab, a humanized monoclonal antibody against folate receptor α, in epithelial ovarian cancer: a phase i study. Clinical Cancer Research. 2010;16(21):5288–95. https://doi.org/10.1158/1078-0432.Ccr-10-0700.
Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor α in oncology. Nature Reviews Clinical Oncology. 2020;17(6):349–59. https://doi.org/10.1038/s41571-020-0339-5.
Tang F, Tie Y, Tu C, Wei X. Surgical trauma-induced immunosuppression in cancer: recent advances and the potential therapies. Clin Transl Med. 2020;10(1):199–223. https://doi.org/10.1002/ctm2.24.
Au KK, Le Page C, Ren R, Meunier L, Clement I, Tyrishkin K, Peterson N, Kendall-Dupont J, Childs T, Francis JA, Graham CH, Craig AW, Squire JA, Mes-Masson AM, Koti M. Stat1-associated intratumoural th1 immunity predicts chemotherapy resistance in high-grade serous ovarian cancer. J Pathol Clin Res. 2016;2(4):259–70. https://doi.org/10.1002/cjp2.55.
Wu Y, Tian Z, Wei H. Developmental and functional control of natural killer cells by cytokines. Front Immunol. 2017;8:930. https://doi.org/10.3389/fimmu.2017.00930.
Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, Du VY, Schlessinger J, Goldberg SB, Chiang A, Sanmamed MF, Melero I, Agorreta J, Montuenga LM, Lifton R, et al. Impaired hla class i antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7(12):1420–35. https://doi.org/10.1158/2159-8290.CD-17-0593.
Poznanski SM, Nham T, Chew MV, Lee AJ, Hammill JA, Fan IY, Butcher M, Bramson JL, Lee DA, Hirte HW, Ashkar AA. Expanded cd56(superbright)cd16(+) nk cells from ovarian cancer patients are cytotoxic against autologous tumor in a patient-derived xenograft murine model. Cancer Immunol Res. 2018;6(10):1174–85. https://doi.org/10.1158/2326-6066.CIR-18-0144.
Maas RJ, Hoogstad-van Evert JS, Van der Meer JM, Mekers V, Rezaeifard S, Korman AJ, de Jonge PK, Cany J, Woestenenk R, Schaap NP, Massuger LF, Jansen JH, Hobo W, Dolstra H. Tigit blockade enhances functionality of peritoneal nk cells with altered expression of dnam-1/tigit/cd96 checkpoint molecules in ovarian cancer. Oncoimmunology. 2020;9(1):1843247. https://doi.org/10.1080/2162402X.2020.1843247.
Bareche Y, Pommey S, Carneiro M, Buisseret L, Cousineau I, Thebault P, Chrobak P, Communal L, Allard D, Robson SC, Mes-Masson AM, Provencher D, Lapointe R, Stagg J. High-dimensional analysis of the adenosine pathway in high-grade serous ovarian cancer. J Immunother Cancer. 2021;9(3). https://doi.org/10.1136/jitc-2020-001965.
S.M. P, Singh K, Ritchier TM, Aguiar JA, Fan IY, Portillo AL, Rojas EA, Vahaedi F, El-Sayes A, Xing S, Butcher M, Lu Y, Doxey AC, Schertzer JD, Hirte HW, Ashkar AA. Metabolic flexibility determines human nk cell functional fate in the tumor microenvironment. Cell Metab. 2021;33:1–16. https://doi.org/10.1016/j.cmet.2021.03.023.
Klose R, Krzywinska E, Castells M, Gotthardt D, Putz EM, Kantari-Mimoun C, Chikdene N, Meinecke A-K, Schrödter K, Helfrich I, Fandrey J, Sexl V, Stockmann C. Targeting vegf-a in myeloid cells enhances natural killer cell responses to chemotherapy and ameliorates cachexia. Nature Communications. 2016;7(1):12528. https://doi.org/10.1038/ncomms12528.
Curtarello M, Tognon M, Venturoli C, Silic-Benussi M, Grassi A, Verza M, Minuzzo S, Pinazza M, Brillo V, Tosi G, Ferrazza R, Guella G, Iorio E, Godfroid A, Sounni NE, Amadori A, Indraccolo S. Rewiring of lipid metabolism and storage in ovarian cancer cells after anti-vegf therapy. Cells. 2019;8(12). https://doi.org/10.3390/cells8121601.
Sheppard S, Santosa EK, Lau CM, Violante S, Giovanelli P, Kim H, Cross JR, Li MO, Sun JC. Lactate dehydrogenase a-dependent aerobic glycolysis promotes natural killer cell anti-viral and anti-tumor function. Cell Rep. 2021;35(9):109210. https://doi.org/10.1016/j.celrep.2021.109210.
Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, Rojas EA, Vahedi F, El-Sayes A, Xing S, Butcher M, Lu Y, Doxey AC, Schertzer JD, Hirte HW, Ashkar AA. Metabolic flexibility determines human nk cell functional fate in the tumor microenvironment. Cell Metabolism. 2021;33(6):1205–1220.e5. https://doi.org/10.1016/j.cmet.2021.03.023.
Hodeib M, O’Grodzinski MP, Vergnes L, Reue K, Karlan BY, Lunt SY, Aspuria PJ. Metformin induces distinct bioenergetic and metabolic profiles in sensitive versus resistant high grade serous ovarian cancer and normal fallopian tube secretory epithelial cells. Oncotarget. 2018;9:4044–60.
Chae C-S, Teran-Cabanillas E, Cubillos-Ruiz JR. Dendritic cell rehab: new strategies to unleash therapeutic immunity in ovarian cancer. Cancer Immunology, Immunotherapy. 2017;66(8):969–77. https://doi.org/10.1007/s00262-017-1958-2.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (time) for effective therapy. Nat Med. 2018;24(5):541–50. https://doi.org/10.1038/s41591-018-0014-x.
Krockenberger M, Kranke P, Häusler S, Engel JB, Horn E, Nürnberger K, Wischhusen J, Dietl J, Hönig A. Macrophage migration-inhibitory factor levels in serum of patients with ovarian cancer correlates with poor prognosis. Anticancer Res. 2012;32(12):5233–8.
Zucha MA, Wu ATH, Lee WH, Wang LS, Lin WW, Yuan CC, Yeh CT. Bruton’s tyrosine kinase (btk) inhibitor ibrutinib suppresses stem-like traits in ovarian cancer. Oncotarget. 2015;6(15):13255–68. https://doi.org/10.18632/oncotarget.3658.
Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, Boylan KLM, Wong HC, Skubitz APN, Miller JS, Geller MA. Il-15 super-agonist (alt-803) enhances natural killer (nk) cell function against ovarian cancer. Gynecol Oncol. 2017;145(3):453–61. https://doi.org/10.1016/j.ygyno.2017.02.028.
Becker-Hapak MK, Shrestha N, McClain E, Dee MJ, Chaturvedi P, Leclerc GM, Marsala LI, Foster M, Schappe T, Tran J, Desai S, Neal CC, Pence P, Wong P, Wagner JA, Russler-Germain D, Zhu X, Spanoudis CM, Gallo VL, et al. A fusion protein complex that combines il12, il15, and il18 signaling to induce memory-like nk cells for cancer immunotherapy. Cancer Immunology Research. 2021;1002(2020). https://doi.org/10.1158/2326-6066.Cir-20-1002.
Miller EM, Samec TM, Alexander-Bryant AA. Nanoparticle delivery systems to combat drug resistance in ovarian cancer. Nanomedicine. 2021;31:102309. https://doi.org/10.1016/j.nano.2020.102309.
Kim KS, Kim DH, Kim DH. Recent advances to augment nk cell cancer immunotherapy using nanoparticles. Pharmaceutics. 2021;13(4). https://doi.org/10.3390/pharmaceutics13040525.
Marchetti C, Palaia I, Giorgini M, De Medici C, Iadarola R, Vertechy L, Domenici L, Di Donato V, Tomao F, Muzii L, Benedetti Panici P. Targeted drug delivery via folate receptors in recurrent ovarian cancer: a review. Onco Targets Ther. 2014;7:1223–36. https://doi.org/10.2147/OTT.S40947.
Li X, McTaggart M, Malardier-Jugroot C. Synthesis and characterization of a ph responsive folic acid functionalized polymeric drug delivery system. Biophys Chem. 2016;214-215:17–26. https://doi.org/10.1016/j.bpc.2016.04.002.
Son S, Rao NV, Ko H, Shin S, Jeon J, Han HS, Nguyen VQ, Thambi T, Suh YD, Park JH. Carboxymethyl dextran-based hypoxia-responsive nanoparticles for doxorubicin delivery. Int J Biol Macromol. 2018;110:399–405. https://doi.org/10.1016/j.ijbiomac.2017.11.048.
Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D, Morosky A, Yang P, Ruman J, Matei D. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of keynote-028. Gynecol Oncol. 2019;152(2):243–50. https://doi.org/10.1016/j.ygyno.2018.11.017.
Bösmüller HC, Wagner P, Pham DL, Fischer AK, Greif K, Beschorner C, Sipos B, Fend F, Staebler A. Cd56 (neural cell adhesion molecule) expression in ovarian carcinomas: association with high-grade and advanced stage but not with neuroendocrine differentiation. Int J Gynecol Cancer. 2017;27(2):239–45. https://doi.org/10.1097/igc.0000000000000888.
Tinker AV, Hirte HW, Provencher D, Butler M, Ritter H, Tu D, Azim HA, Paralejas P, Grenier N, Hahn S-A, Ramsahai J, Seymour L. Dose-ranging and cohort-expansion study of monalizumab (iph2201) in patients with advanced gynecologic malignancies: a trial of the Canadian Cancer Trials Group (CCTG): Ind221. Clinical Cancer Research. 2019;25(20):6052–60. https://doi.org/10.1158/1078-0432.Ccr-19-0298.
Andre P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Blery M, Bonnafous C, Gauthier L, Morel A, Rossi B, Remark R, Breso V, Bonnet E, Habif G, Guia S, Lalanne AI, Hoffmann C, Lantz O, et al. Anti-nkg2a mab is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both t and nk cells. Cell. 2018;175(7):1731–1743.e13. https://doi.org/10.1016/j.cell.2018.10.014.
Zaghi E, Calvi M, Marcenaro E, Mavilio D, Di Vito C. Targeting nkg2a to elucidate natural killer cell ontogenesis and to develop novel immune-therapeutic strategies in cancer therapy. Journal of Leukocyte Biology. 2019;105(6):1243–51. https://doi.org/10.1002/JLB.MR0718-300R.
Tinker AV, Hirte HW, Provencher D, Butler M, Ritter H, Tu D, Azim HA Jr, Paralejas P, Grenier N, Hahn SA, Ramsahai J, Seymour L. Dose-ranging and cohort-expansion study of monalizumab (iph2201) in patients with advanced gynecologic malignancies: a trial of the Canadian Cancer Trials Group (CCTG): Ind221. Clin Cancer Res. 2019;25(20):6052–60. https://doi.org/10.1158/1078-0432.CCR-19-0298.
Portillo AL, Hogg R, Poznanski SM, Rojas EA, Cashell NJ, Hammill JA, Chew MV, Shenouda MM, Ritchie TM, Cao QT, Hirota JA, Dhesy-Thind S, Bramson JL, Ashkar AA. Expanded human nk cells armed with car uncouple potent anti-tumor activity from off-tumor toxicity against solid tumors. iScience. 2021;24(6). https://doi.org/10.1016/j.isci.2021.102619.
Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like nk cells. Blood. 2012;120(24):4751–60. https://doi.org/10.1182/blood-2012-04-419283.
Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee YS, Mulder A, Claas F, Cooper MA, Fehniger TA. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123. https://doi.org/10.1126/scitranslmed.aaf2341.
Garcia-Martinez E, Redondo A, Piulats JM, Rodriguez A, Casado A. Are antiangiogenics a good ‘partner’ for immunotherapy in ovarian cancer? Angiogenesis. 2020;23(4):543–57. https://doi.org/10.1007/s10456-020-09734-w.
Garcia A, Singh H. Bevacizumab and ovarian cancer. Ther Adv Med Oncol. 2013;5(2):133–41. https://doi.org/10.1177/1758834012467661.
Acknowledgements
We gratefully acknowledge Designs that Cell for assistance with our figure. The Dalhousie University is located in Mi’kma’ki, the ancestral and unceded territory of the Mi’kmaq.
Funding
This research was supported by a Terry Fox Research Institute New Investigator award to JEB within the pan-Canadian Immunotherapeutic Network, and research funding from Ovarian Cancer Canada to JEB and BML. SN and MPT are trainees in the Cancer Research Training Program of the Beatrice Hunter Cancer Research Institute, with funds for MPT provided by the Bruce and Dorothy Rosetti Endowment through the Dalhousie Medical Research Foundation. MPT is supported by a Nova Scotia Graduate Scholarship. BioCanRx, a Canadian Network of Centres of Excellence, provided funding support for this research to APN. SN is supported by a Killam Scholarship, the President’s award from Dalhousie University and a CIHR Vanier Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Pugh-Toole, M., Nicolela, A.P., Nersesian, S. et al. Natural Killer Cells: the Missing Link in Effective Treatment for High-Grade Serous Ovarian Carcinoma. Curr. Treat. Options in Oncol. 23, 210–226 (2022). https://doi.org/10.1007/s11864-021-00929-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-021-00929-x